Superior Canal Dehiscence Patients Have Smaller Mastoid Volume than Age- and Sex-Matched Otosclerosis and Temporal Bone Fracture Patients
Article information
Abstract
Background and Objectives
The purpose of the study was to compare the mastoid air-cell volume of the patients with superior semicircular canal dehiscence syndrome (SCDS) and that of the control patients with otosclerosis and temporal bone (TB) fracture.
Subjects and Methods
Ten patients with SCDS were enrolled and 10 patients with bilateral otosclerosis and TB fracture were selected as control groups by age and sex matching. To measure the mastoid air-cell volume, 3D reconstruction software was used.
Results
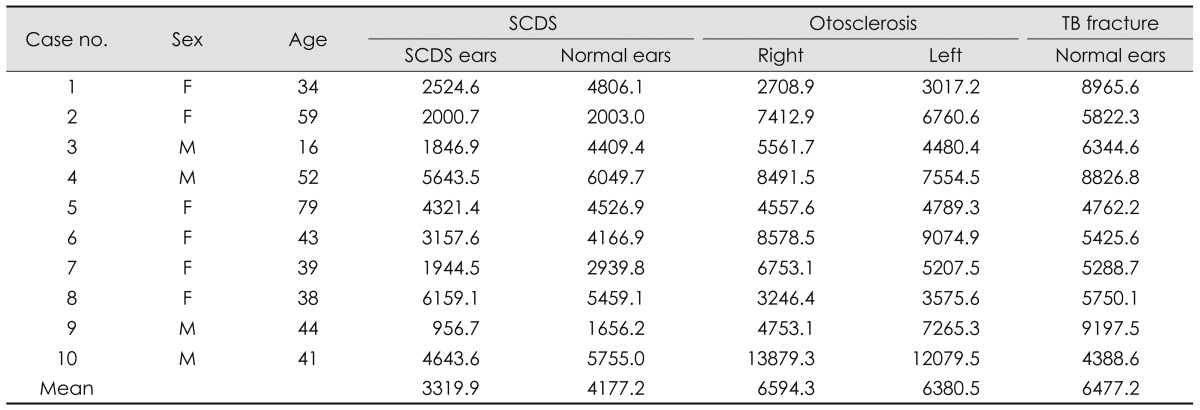
In 10 patients with SCDS, the mean age was 44.5 years, ranging from 16 to 79 years (M : F=4 : 6). Mean mastoid air-cell volume in the SCDS side was 3319.9 mm3, whereas 4177.2 mm3 in the normal side (p=0.022). Mean mastoid air-cell volume in the right side of otosclerosis patients was 6594.3 mm3 and it was not different from 6380.5 mm3 in the left side (p=0.445). Mean mastoid air-cell volume in normal side of TB fracture was 6477.2 mm3. The mastoid air-cell volume in the SCDS side was significantly smaller than that of otosclerosis and TB fracture patients (p=0.009, p=0.002, respectively). The mastoid air-cell volume in the normal side of SCDS was significantly smaller than that of TB fracture (p=0.019), but not significant with that of otosclerosis (p=0.063).
Conclusions
Our findings revealed that the mastoid air-cell volume in the SCDS side was significantly smaller than control group, which suggest that the decreased mastoid pneumatization is closely related to the generation of SCDS.
Introduction
Superior semicircular canal dehiscence syndrome (SCDS) is a recently recognized clinical condition, which was initially described by Minor, et al.1) The syndrome usually encompasses a constellation of vestibular and audiological symptoms, such as sound and/or pressure induced vertigo and oscillopsia, along with conductive hearing loss and autophony, and typically manifests as sound and/or pressure induced nystagmus at the plane of the superior semicircular canal (SSC).2-4)
The proposed underlying mechanism involves the existence of a dehiscence at the apex of the SSC (third mobile window), in addition to the round and oval windows of the osseous cochlea, which in effect potentiates the transmission of sudden changes in the middle and/or intracranial pressure, thus altering the related neural firing rates of the vestibular system, and may also alter inner ear fluid dynamics, causing dissemination of the acoustic energy.5,6)
While the classic presentation can be suspected on clinical and audiometric data, imaging plays an important role in the evaluation of these patients. Advances in computed tomography (CT) now allow high resolution images to demonstrate the bony defect, while multiplanar reformations can also aid in the radiologic diagnosis.7-9)
Despite the several investigations on this condition, there are no clear causes of SCDS. Certainly, a congenital/developmental basis for the condition has been described.9-13) There was a report describing the development of SCDS from an intact ear, confirmed by serial follow-up CT scans.14) In that study, osteomyelitis and increased bony absorption associated with chronic brain pulsation might be one of the possible causes of SCDS. Because inflammatory process is related to the decreased mastoid air-cell volume,15,16) we wanted to compare the mastoid air-cell volume of the patients with SCDS and that of the age- and sex-matched patients with otosclerosis and temporal bone (TB) fracture, who are supposed to represent normal mastoid pneumatization.
Subjects and Methods
10 patients with SCDS confirmed by symptoms, signs, vestibular evoked myogenic potential and TB CT imaging were enrolled. To compare the mastoid air-cell volume, the otosclerosis and TB fracture patients identified in the TB CT were selected as control groups. The selection was made by age and sex matching. In the control groups, the otosclerosis was identified in both sides, and with no other pathology, such as chronic otitis media (COM) and middle ear effusion. This study was approved by the Institutional Review Board of our institute and followed the recommended guidelines.
High-resolution TB CT scans were performed using a 64 row detector CT scanner (Siemens, Medical System, Erlanger, Germany). Images were helically acquired without contrast and were reconstructed using a bone plus algorithm. The axial plane images were acquired using a 0.6 mm section thickness at 200 mA and 120.0 kV. The coronal and oblique sagittal reconstruction images, which were parallel to the superior semicircular canal, were acquired using 0.4 mm interval reconstructions. All of the images were evaluated on a Diagnostic Petavision pacs station (Emsoma, Seoul, Korea). To measure the mastoid air-cell volume, we used 3D reconstruction software. Continuous non-overlapping sections of TB CT scan images were used. The imaging data were stored in a Digital Imaging and Communication in Medicine file and then imported to a personal computer running Vworks 4.0 software (Cybermed, Seoul, Korea). When performing reconstruction using a surface rendering algorithm, the selection of the window thresholds were -1024 to -318 Hounsfield Units. The volume of mastoid air-cell was automatically calculated in the 3D reconstruction (Fig. 1).

Three-dimensional reconstruction of computed tomography scans was performed using a surface rendering algorithm. The volume of mastoid air-cell was automatically calculated by software.
Statistical analysis was conducted using Wilcoxon signed rank test to compare volume between the SCDS side and the normal side in SCDS patients, and left and right side of the otosclerosis patients. The differences of mastoid air-cell volume in the SCDS, otosclerosis and TB fracture were determined using the Kruskal-Wallis test and Mann-Whitney U test. All statistical analyses were performed using SPSS 20.0 software (SPSS, IBM corp., Armonk, NY, USA), and the p values <0.05 were considered significant.
Results
Ten patients with SCDS were enrolled. Sex ratio was 4 : 6 (male : female). The mean age was 44.5 years, ranging from 16 to 79 years. The sides of SCDS were right in 4 and left in 6.
Mean mastoid air-cell volume in SCDS side was 3319.9 mm3, whereas 4177.2 mm3 in the normal side (Table 1). There was a significant difference of the mastoid air-cell volume between the lesion side and the normal side of SCDS patients (p=0.022, Wilcoxon signed rank test).
The mastoid air-cell volume (mm3) in patients with superior semicircular canal dehiscence syndrome, otosclerosis and temporal bone fracture
Mean mastoid air-cell volume of otosclerosis patients was 6594.3 mm3 in the right side and 6380.5 mm3 in the left side (Table 1). And there was no significant difference of mastoid air-cell volume between the right and left sides (p=0.445, Wilcoxon signed rank test). Mean mastoid air-cell volume in normal side of TB fracture was 6477.2 mm3 (Table 1). There is no difference between the mastoid air-cell volume of the otosclerosis and that of TB fracture patients (p=0.684).
The mastoid air-cell volume of the SCDS (both sides) showed significant difference compared to that in any side of the otosclerosis patients (p=0.013, Kruskal-Wallis test). In the post-hoc analysis, the mastoid air-cell volume in SCDS side was significantly smaller than those of otosclerosis patients (p=0.009, Mann-Whitney U test)(Fig. 2). However, the difference between the mastoid air-cell volume in the normal side of SCDS patients and that of otosclerosis patients was not significant (p=0.063, Mann-Whitney U test).

Comparison of the mastoid air-cell volumes between the superior canal dehiscence syndrome (lesion and normal side), temporal bone fracture (normal side) and otosclerosis (right-side) patients. *p=0.009, †p=0.002, ‡p=0.063, §p=0.019 in Mann-Whitney U test.
Similarly, the mastoid air-cell volume of the SCDS (both sides) showed significant difference compared to that in TB fracture patients (p=0.006, Kruskal-Wallis test). In the post-hoc analysis, the mastoid air-cell volume in SCDS side and normal side of SCDS patients were significantly smaller than that of TB fracture patients (p=0.002, p=0.019, respectively, Mann-Whitney U test)(Fig. 2).
Discussion
The decrease of mastoid air-cell volume could be seen several conditions, such as otitis media with effusion, COM, and cholesteatoma.15,16) Many studies reported that the inflammatory process is related to the decreased mastoid air-cell volume. Our results demonstrated significantly smaller mastoid air-cell volume in the SCDS side than in that of the otosclerosis patients and TB fracture patients (p=0.009, p=0.002, respectively). The mastoid air-cell volume (4177.2 mm3) in the normal side of SCDS patients was smaller than that (6594.3 mm3 in the right ear and 6380.5 mm3 in the left ear) of the otosclerosis patients, although it was not significant (p=0.063). But, the mastoid air-cell volume in the normal side of SCDS patients significantly smaller than TB fracture patients (4177.2 mm3 vs. 6477.2 mm3, p=0.019). Because no patients with SCDS in this study had soft tissue density in the mastoid in TB CT scans, suggesting there is no active inflammation in the mastoid, inflammatory process (acute otitis media or middle ear effusion) in the early childhood could have had a role in the decreased mastoid air-cell volume in the SCDS ear.
It remains unclear whether SCDS is a congenital/developmental disorder or whether it is acquired. It has been reported that TB specimens from infants show uniformly thin bone over the superior canal in the middle fossa at birth, with gradual thickening until 3 years of age, and that SCDS may arise from failure of postnatal bone development.9) Tsunoda and Terasaki17) has described the embryological basis for dehiscence. The precartilage bordering the developing membranous labyrinth dedifferentiates into loose reticular mesenchyme. If the otocyst is situated close to the developing brain during this stage, there is inadequate space for the growth of the superior surface of the superior semicircular canal as the otocyst may lie against the dura. Hindering gradual thickening of the superior canal roof by otitis media before the age of 3, associated with the decreased mastoid pneumatization, may make the superior canal roof thin enough. Additionally, SCDS may be generated by chronic brain pulsation and the pressure exerted by the temporal lobe on the middle cranial fossa.9)
However, another study demonstrated that the radiologic prevalence of SCDS among older age groups increases, suggesting that SCDS is more commonly an acquired rather than developmental condition.18) Chronic bone inflammation by previous otitis media in childhood, which can be identified as having decreased mastoid volume, may render the bone overlying the superior canal susceptible to brain pulsation or the pressure from the temporal lobe, resulting in the generation of SCDS.
Conclusion
Our findings revealed that the mastoid air-cell volume in the SCDS side was significantly smaller than that of otosclerosis and TB fracture patients, furthermore the volume in the normal side of SCDS patients was significantly smaller than that of TB fracture patients, which suggest that the decreased mastoid pneumatization is closely related to the generation of SCDS, whether SCDS is a congenital/developmental disorder or whether it is acquired.