 |
 |
- Search
| J Audiol Otol > Volume 17(2); 2013 > Article |
Abstract
Background and Objectives
Gingko biloba extract is known for enhancing blood circulation, scavenging free radicals, and antagonizing against platelet-activating factor. This study evaluated the effect of Gingko biloba on the noise-induced temporary threshold shift of hearing.
Materials and Methods
Temporary threshold shift was induced by exposing mice to 110 dB SPL sound for 1 hour. The experimental group consisted of mice fed Gingko biloba [3 mg/kg, 6 mg/kg, and 12 mg/kg in 0.5% carboxymethyl cellulose (CMC)] for 7 days before noise exposure. CMC solution without Gingko biloba was fed to control mice. Hearing threshold was measured by auditory brainstem response (ABR) and distortion product otoacoustic emission (DPOAE).
Results
The hearing threshold increased after noise exposure and recovered to normal within 5 days in all groups. Compared to control mice (fed CMC solution only), mice fed Gingko biloba showed more rapid recovery of ABR threshold at 16 kHz in all three experimental groups. At the other frequencies, there was no significant change in hearing recovery in the Gingko biloba groups. There was no difference in DPOAE between groups.
Gingko leaf is a medicinal herb that has been used in the Oriental world, including China, and is now being used for many purposes worldwide, including in the United States and Europe in the form of a drug made from Gingko biloba extract. The main actions of Gingko leaves are thought to be generated by flavonoid glycosides. Representative pharmacological actions are the improvement of blood and oxygen supply in the central and peripheral systems by free radical scavenging, vasodilation, and inhibition of platelet-activating factor. Clinically, Gingko biloba has been proven effective for Alzheimer's disease, cerebral insufficiency, cognitive disorders, tinnitus, and dizziness.1-4)
Previous research on Gingko biloba indicates that it can improve hearing loss, especially noise-induced hearing loss. Lamm and Arnold5) administered Gingko biloba (8.75 mg/kg) to a guinea pig exposed to intense noise and measured cochlear blood flow and perilymphatic oxygen pressure (PO2) 3 hours before and after noise exposure. There was no significant change due to Gingko biloba. However, they administered the drug once, 30 minutes after noise exposure, at one dose level, and the observation period was just 2 hours. Accordingly, there is a need to study the effect of Gingko biloba under various conditions and with various doses.
In this study, we observed hearing changes after oral administration of Gingko biloba in noise-exposed mice to determine whether Gingko biloba has a protective effect against noise-induced hearing loss.
We used 4 week-old CBA mice that tested normal for Preyer's reflex and auditory brainstem response (ABR).
The booth to generate noise was soundproof. We connected a speaker (290-8L, ALTEC LANSING, Oklahoma City, OK, USA) and an amplifier (R-399, INTER M, Seoul, Korea) with input and output resistances of 8 Ω, respectively. The amplifier was placed in the left corner of the noise booth with a speaker on it, and the horn was attached at an angle of 45 degrees.
Zolazepam/tiletamine (Zoletil) 25 mg/kg and xylazine (Rompun) 10 mg/kg were administered intraperitoneally for anesthesia, and half of the above amounts were added if necessary.
We exposed mice with normal hearing to broad band white noise of 110 dB SPL for 1 hour to induce TTS. We administered Gingko biloba once a day for 7 days before noise exposure, and the dose was set to the adult standard dose, i.e., 3 mg/kg powder. The animals were divided into four groups, a control group that did not receive Gingko biloba, a standard dose group, a double (6 mg/kg) dose group, and a quadruple (12 mg/kg) dose group. For each experiment, we used one animal as a control and three animals in each remaining group. Then, we repeated the experiment three times using the same experimental protocol; therefore, we used three control animals and nine animals in each of the other groups.
We used SK Chemical's Gingko leaf extract powder products and mixed them with 0.5% carboxymethyl cellulose solution.
For measurement of hearing level, we used ABR and distortion-product otoacoustic emission (DPOAE) with an auditory evoked potential workstation (Tucker-Davis Technologies, Alachua, FL, USA). Tone burst stimulation was used to measure frequency-specific ABR. The frequencies measured were 4, 8, 16, and 32 kHz, and we gained the waveform by repeatedly decreasing the stimulation tone by 5 dB from the strength of 90 dB HL. The most uniformly generated waveform was used for analysis. We judged a waveform with an amplitude over 0.2 µV that appeared to be similar to the reaction from the previous stimulation tone as a significant waveform. We determined the minimum stimulation level with a significant signal as the hearing threshold and then calculated the hearing threshold shift before and after noise exposure. DPOAE was measured using an amplifier system that could provide two stimulation tones. We generated f1 and f2 primary stimulation tones (f2/f1=1.2) by using a dual channel synthesizer. Sounds were introduced to a mouse through an acoustic probe (ER-10B+) attached to the external auditory meatus. The emission sound from the microphone attached to the probe was collected at the speed of 44100 Hz and analyzed with a 4096 point fast Fourier transform. For the measurement of frequency-specific responses, we set the F2 stimulation tone to 4, 5.6, 8, 11.3, and 16 kHz. Threshold was determined when DPOAE response exceeded the noise level on the measured graph at each frequency.
All the measured values were statistically validated with the t-test. A p value less than 0.05 was considered significant.
Both the experimental groups and the control group showed a threshold increase of hearing level immediately after noise exposure, and began to slowly recover. The ABR threshold in the control group increased at all frequencies after noise exposure, showing a large difference from the resting hearing threshold, but it recovered to the resting hearing level by day 5. In the Gingko biloba groups, the hearing level increased after noise exposure and recovered to the resting level at 4 kHz. The hearing levels at both 8 kHz and 16 kHz of the 6 mg/kg and 12 mg/kg groups were better than the hearing level of the control group at day 3 after noise exposure. The hearing threshold at 32 kHz was different only in the 6 mg/kg group on day 1 after exposure. On day 7 after exposure, groups that received the drug showed no difference from the control group at all frequencies (Fig. 1).
The DPOAE thresholds of Gingko biloba groups were better than that of the control group on day 7 after noise exposure at 4 kHz. However, there were no statistical differences in hearing levels among groups at other frequencies (Fig. 2).
Many theories have been suggested for the pathology of noise-induced inner ear damage, and drug administration experiments and treatments are conducted on the basis of such theories. A decrease of cochlear blood flow with hypoxia-induced oxidative stress and permanent hearing loss have been proposed for a long time. This concept proposes that blood flow into the inner ear is reduced by noise and, subsequently, the inner ear becomes deprived of oxygen. A mechanism involving an increase of oxygen consumption in the tissue without regard to blood flow decrease has also been suggested. Mitochondrial dysfunction, excitotoxicity due to glutamate increase, and reduction of glutathione are known to be involved in the pathologic process leading to hypoxia-induced inner ear damage. On the basis of research on the hypoxia-related damage mechanism, various kinds of drug treatments have been tested: acetyl-L-carnitine helping mitochondria function to resist hypoxia, carbamathione acting as a glutamate antagonist of the NMDA receptor, and D-methionine inducing glutathione supplementation.6)
A relationship between noise-induced hearing loss and reactive oxygen species (ROS) was supported by the generation of ROS in noise-exposed mice and prevention of hearing loss by the use of an anti-ROS agent. Also, the last phase of ROS-initiated inner ear damage is known as apoptosis.7)
Encouraged by these theories, anti-ROS agents are actively being studied, such as glutathione, the xanthine oxidase blocker allopurinol, N-L-acetylcysteine, and GSH peroxidase and superoxide dismutase. Animal experiments have been conducted, and clinical research will be conducted in the future.8-10)
Gingko biloba extract is known to be effective for the improvement of central and peripheral blood supply and oxygen transfer, so it was expected to prevent or improve noise-induced hearing loss. In this study, however, the hearing threshold in the Gingko group was better than in the control group at 8 kHz and 16 kHz on day 3 of noise exposure, but the hearing threshold began changing in the same manner in the control group. Such a result can be considered to indicate that Gingko biloba extract administration at 6 mg/kg or 12 mg/kg is effective for early recovery of hearing after noise exposure. However, we cannot prove a protective effect of Gingko biloba because all hearing was recovered on day 7. Nevertheless, we can suggest that the dose of Gingko biloba is related to the recovery of hearing because there was no early recovery of hearing in the 3 mg/kg group. The early recovery of hearing is associated with the fine structure of the internal ear and cochlear nerves, so these tissues should be further studied.
In addition, there was no difference in DPOAE between groups except at 4 kHz on day 7. Therefore, the cochlear hair cells may not be affected by the administration of Gingko biloba.
This research used three doses of Gingko biloba, and the administration period was only one week. Because of these limitations, the effects of long-term or high-dose Gingko biloba should be studied further, and the effect on an animal model with permanent threshold shift should be followed.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST)(2010-0023182).
References
1. Birks J, Grimley EV, Van Dongen M. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev 2002;CD003120PMID: 12519586.


2. Carlson JJ, Farquhar JW, DiNucci E, Ausserer L, Zehnder J, Miller D, et al. Safety and efficacy of a ginkgo biloba-containing dietary supplement on cognitive function, quality of life, and platelet function in healthy, cognitively intact older adults. J Am Diet Assoc 2007;107:422–432. PMID: 17324660.


3. Haguenauer JP, Cantenot F, Koskas H, Pierart H. [Treatment of equilibrium disorders with Ginkgo biloba extract. A multicenter double-blind drug vs. placebo study]. Presse Med 1986;15:1569–1572. PMID: 2947102.

4. Morgenstern C, Biermann E. The efficacy of Ginkgo special extract EGb 761 in patients with tinnitus. Int J Clin Pharmacol Ther 2002;40:188–197. PMID: 12051570.


5. Lamm K, Arnold W. The effect of blood flow promoting drugs on cochlear blood flow, perilymphatic pO(2) and auditory function in the normal and noise-damaged hypoxic and ischemic guinea pig inner ear. Hear Res 2000;141:199–219. PMID: 10713508.


6. Kopke RD, Coleman JK, Liu J, Campbell KC, Riffenburgh RH. Candidate's thesis: enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope 2002;112:1515–1532. PMID: 12352659.


7. Ohlemiller KK, Dugan LL. Elevation of reactive oxygen species following ischemia-reperfusion in mouse cochlea observed in vivo. Audiol Neurootol 1999;4:219–228. PMID: 10436314.


8. Franzé A, Sequino L, Saulino C, Attanasio G, Marciano E. Effect over time of allopurinol on noise-induced hearing loss in guinea pigs. Int J Audiol 2003;42:227–234. PMID: 12790348.


9. Kopke RD, Weisskopf PA, Boone JL, Jackson RL, Wester DC, Hoffer ME, et al. Reduction of noise-induced hearing loss using L-NAC and salicylate in the chinchilla. Hear Res 2000;149:138–146. PMID: 11033253.


10. Yamasoba T, Nuttall AL, Harris C, Raphael Y, Miller JM. Role of glutathione in protection against noise-induced hearing loss. Brain Res 1998;784:82–90. PMID: 9518561.


Fig. 1
ABR thresholds are increased at all frequencies after noise exposure that recovered by 5 days to control (control n=3, Gingko n=9 at each dose). At 8 and 16 kHz, gingko-treated mice (6 mg/kg and 12 mg/kg) showed a better hearing level at 3 days after noise exposure. At 32 kHz, mice treated with gingko (6 mg/kg) showed a better hearing level one day after noise exposure. At the other frequencies, the gingko-treated group did not show a significant change of thresholds (*p<0.05 compared to the control group). ABR: auditory brainstem response.
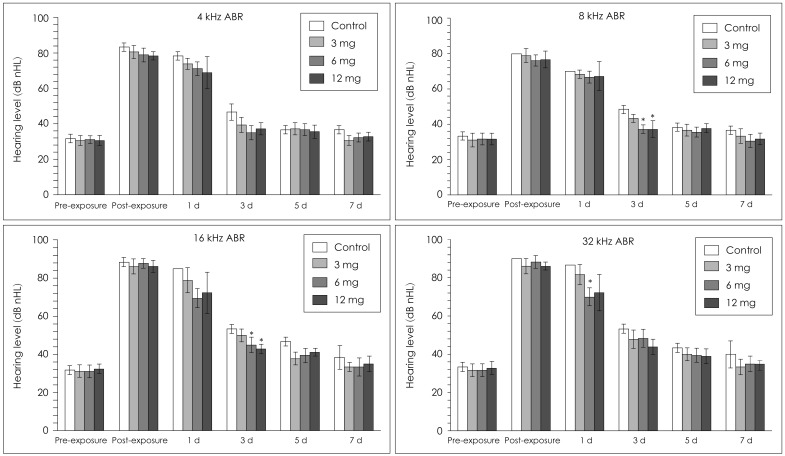
Fig. 2
DPOAE thresholds are increased at all frequencies after noise exposure that recovered by 5 days to control (control n=3, Gingko n=9 at each dose). The gingko-treated group shows a better threshold at 7 days after noise exposure at 4 kHz. At the other frequencies, there was no significant difference between experimental and control groups (*p<0.05 compared to the control). DPOAE: distortion product otoacoustic emission.







