Effects of an Auditory Lateralization Training in Children Suspected to Central Auditory Processing Disorder
Article information
Abstract
Background and Objectives
Central auditory processing disorder [(C)APD] refers to a deficit in auditory stimuli processing in nervous system that is not due to higher-order language or cognitive factors. One of the problems in children with (C)APD is spatial difficulties which have been overlooked despite their significance. Localization is an auditory ability to detect sound sources in space and can help to differentiate between the desired speech from other simultaneous sound sources. Aim of this research was investigating effects of an auditory lateralization training on speech perception in presence of noise/competing signals in children suspected to (C)APD.
Subjects and Methods
In this analytical interventional study, 60 children suspected to (C)APD were selected based on multiple auditory processing assessment subtests. They were randomly divided into two groups: control (mean age 9.07) and training groups (mean age 9.00). Training program consisted of detection and pointing to sound sources delivered with interaural time differences under headphones for 12 formal sessions (6 weeks). Spatial word recognition score (WRS) and monaural selective auditory attention test (mSAAT) were used to follow the auditory lateralization training effects.
Results
This study showed that in the training group, mSAAT score and spatial WRS in noise (p value≤0.001) improved significantly after the auditory lateralization training.
Conclusions
We used auditory lateralization training for 6 weeks and showed that auditory lateralization can improve speech understanding in noise significantly. The generalization of this results needs further researches.
Introduction
Sound localization is one of the most important functions of auditory system in humans and other animals and is mainly achievable by using binaural cues including interaural time difference (ITD)/phase difference and interaural level difference (ILD)/intensity difference. Accurate sound localization in animals is crucial for survival (escaping from a predator, hunting a prey and finding a mate) [12]. In addition, sound localization is an auditory ability to detect sound sources in the space (auditory scene analysis). This type of segregating and grouping of sound sources can help to differentiate between the desired stream of speech and other simultaneous sound sources that can be regarded as noise [34]. Therefore it is one of the important auditory functions for understanding and following target speech in everyday situations [5]. It is known that speech perception in noise or in presence of competing signals is better when target speech and competing signal arrive from different spatial directions [6] and auditory localization in humans forms a base for higher order auditory functions (cocktail party effect) [47].
Central auditory processing disorder [(C)APD] is a deficit in auditory neural processing that is not caused by higher-order language, cognitive or related factors [8]. Earlier identification of (C)APD can lead to a more timely diagnosis, which in turn may assist in a better understanding of the child's poor academic performance. This may also increase the opportunity for appropriate and earlier intervention, thus minimizing educational and other associated deficits and improving everyday listening functions [9]. One of the problems in children with (C)APD is localization/lateralization difficulties or spatial processing disorders (SPDs) [101112]. SPDs have been overlooked in children with (C)APD despite their significance. Dillon and Cameron maintained that a substantial proportion of children with (C)APD suffer from SPD and this may interfere with sound source segregation and understanding of speech in presence of competing sound sources [1314]. It can be especially problematic in classroom [15] where higher signal to noise ratio (SNR) is needed [14] and may lead to academic failure [16].
It is supposed that auditory localization/lateralization training may change children' ability to use spatial clues for segregating target speech from competing signals/noise and improve their speech perception in everyday listening situations. So aim of the present research was investigating effects of an auditory lateralization training on speech perception in presence of competing signals/noise in children suspected to (C) APD. As it is difficult to diagnose children with pure (C)APD, term "suspected to (C)APD" seems more appropriate [17].
Subjects and Methods
In this analytical interventional study, 60 children suspected to (C)APD (40 boys and 20 girls) were selected based on inclusion criteria. All inclusion criteria were same for training and control group and children who met inclusion criteria were randomly divided into two groups: 30 children in the control group (mean age 9.07±1.25 years; 10 females and 20 males) and 30 children in the training group (mean age 9.00±1.28; 10 females and 20 males). Both groups were matched in terms of sex and age. As there is not a gold standard test for (C)APD diagnosis, we selected dichotic digit test (DDT) [18]/ pitch pattern sequence test (PPS) [19]/monaural selective auditory attention test (mSAAT) [20] based on MAPA (multiple auditory processing assessment) test battery [2122]. MAPA study showed that DDT/PPS/mSAAT test battery can provide 90% sensitivity and 100% specificity in (C)APD diagnosis [821]. Before starting study, to establish norms for Persian-version of DDT (free recall) [18], mSAAT-Persian version [20], PPS test [19] and spatial word recognition score in noise test, we conduct a study on 750 students of 8 to 12 years old (mean age 10.00±1.41; 250 males and 500 females). For establishing normative data, inclusion criteria were as follows: normal PTA (auditory threshold less than 20 dB HL in 500 to 4,000 Hz frequency range) in both ears; normal middle ear function (A type tympanogram); 85 or higher Wechsler intelligence quotient (IQ) score, monolingualism (Persian language); no history of attention-deficit hyperactivity disorder (ADHD), seizures, behavioral or developmental disorders; not being on any central nervous system medications; good academic performance. Normative data can be found in result section.
Inclusion criteria for children suspected to (C)APD were as follows: normal PTA (auditory threshold less than 20 dB HL in 500 to 4,000 Hz frequency range) in both ears; symmetric hearing (PTA difference less than 5 dBHL between two ears); normal middle ear function (A type tympanogram); 85 or higher Wechsler IQ score, monolingualism (Persian language); no history of ADHD, seizures, behavioral or developmental disorders; not being on any central nervous system medications; poor academic performance; abnormal results in DDT, PPS and mSAAT. If a child had scores less than 2 standard deviations from established norms in these three tests, he/she was suspected to (C)APD.
Procedure
DDT is composed of naturally spoken digits from 1 to 10 (except for number 4 in Persian language). It requires that 2 number pairs be presented simultaneously to each ear of listeners, and subjects are asked to repeat all 4 numbers regardless of order (free recall). Forty patterns are presented in total. Outcome measure is the percentage of correct responses [18].
PPS test reflects temporal component of auditory pattern recognition. Each item is a set of three pure tones with two different pitches, with a low-frequency tone at 880 Hz and a high-frequency tone at 1,122 Hz. The duration of every tone is 200 ms with 10-ms rise and fall time. These tones are separated by 150-ms intervals and the silence epoch between every set is 6 s. Totally 30 patterns are presented monaurally to each ear. Stimuli were presented at 55 dB SL (re: 1,000 Hz threshold). Outcome of this test is percentage of correct responses [19].
To evaluate speech understanding in presence of competing signals, the Persian version of mSAAT was used. This test is one of the monaural low redundancy tests and it can assess auditory figure-ground skill [23]. This test compares ability of recognizing monosyllabic words (25 words) embedded in a background of story. Both target and competing stimuli are recorded by the same speaker and signal to noise ratio (SNR) is zero dB. Outcome measure is percentage of correct responses for each ear [20].
To evaluate spatial processing, auditory lateralization of monosyllabic words in white noise with zero dB SNR was used. Words were presented randomly through headphones at -90, -60, -30, zero, +30, +60, +90° azimuth (5 words were presented for each location). Test was made by Sound Forge software v8. Word recognition score (WRS) and number of auditory lateralization errors were examined for each spatial location.
Auditory lateralization training including 12 formal sessions (2 sessions in each week) was started in the training group. Each session lasted 45 minutes. A high pass and a low pass noise with 2 kHz cutoff point, with 250 milliseconds duration and 20 milliseconds rise and fall times were used. Stimuli were presented through headphones with 880, 660, 220, zero, -220, -660, -880 microseconds ITDs at 50 dB HL, and the children had to point to the perceived location of sound source [24]. In localization training, loudspeakers are used to make an auditory space around subjects and in lateralization training, sound is delivered through headphones. Headphones are preferable because under headphones, ITD and ILD can be manipulated independently [2526]. These sessions were performed as a game. If the child could point to the correct sound position, he/she received a reward. There were 7 pictures of loudspeakers, in -90, -60, -30, 0, +30, +60, +90° around children. At the beginning of each training session, the administrator participated actively in the lateralization game then gradually the child took over the games and the administrator just gave feedback and rewards based on the child's response.
In the training group, mSAAT and lateralization test were performed again after 12 sessions of lateralization training. For comparison and determining training effects, in the control group, mSAAT and lateralization test were also repeated after 2 months from the first evaluation.
SPSS v21 (IBM, Armonk, NY, USA) was used for statistical data analysis. In addition to descriptive analysis, covariance and Wilcoxon tests were used to show training effects and within group comparisons respectively.
Written consent was received from the parents for evaluation and auditory lateralization sessions. All tests were noninvasive. The control group also received auditory lateralization training after research. Patients' information were kept private.
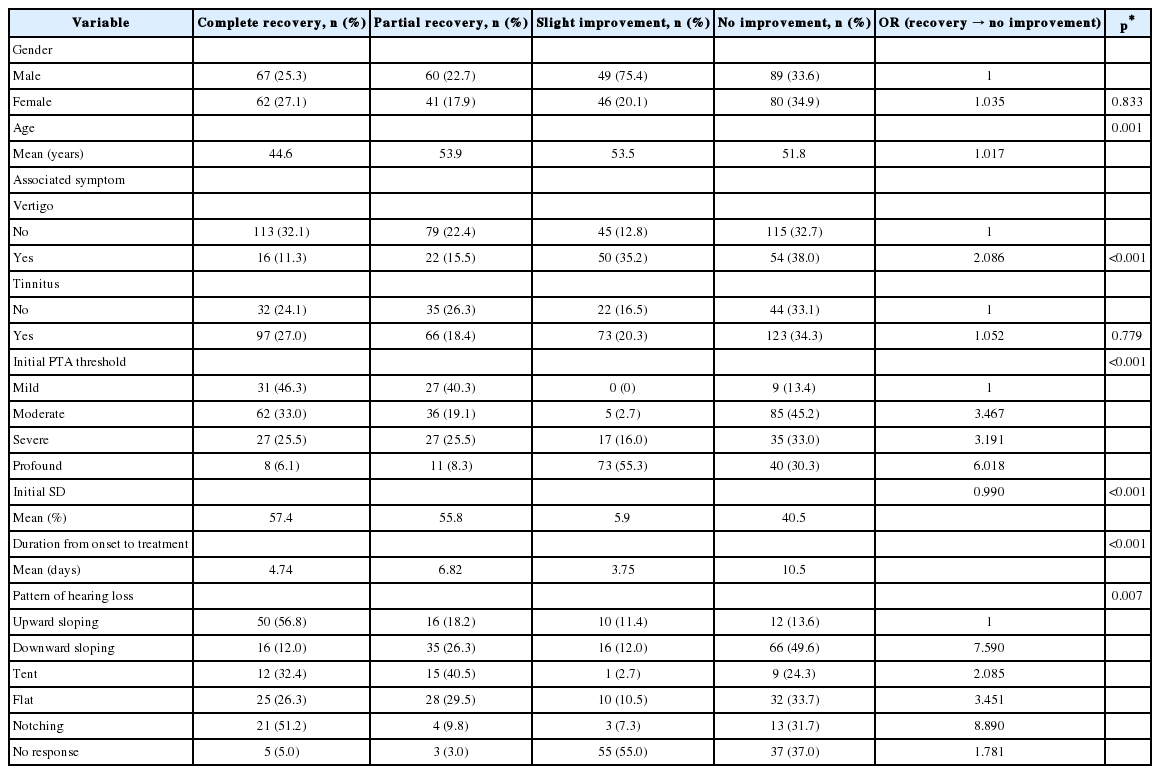
Results
750 normal children (8 to 12 years old; mean age 10.00±1.41 year) including 250 males and 500 females were selected and used for establishing normative data. The means and standard deviations (SDs) of mSAAT-Persian version, Persian version of DDT and PPS test are shown in Table 1. Results of children suspected to (C)APD is also shown in Table 1. As it can be seen children suspected to (C)APD had lower scores in all tests (more than 2 SDs). Means and SDs of number of lateralization errors and spatial WRS in noise for normal children and children suspected to (C)APD are summarized in Table 2. In all spatial directions, children suspected to (C)APD have higher lateralization errors and lower spatial WRS score (more than 2 SDs) than normal children.
Lateralization errors and spatial WRS in noise (percent) scores: normative and (C)APD data (mean±SDs)
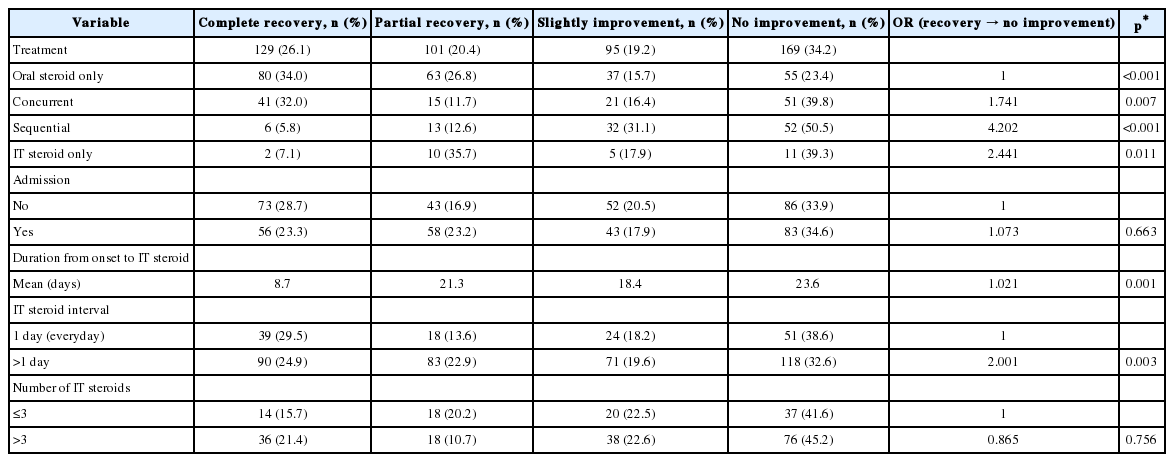
Table 3 shows results of speech understanding in noise (spatial WRS in noise test) and in presence of competing signals (mSAAT) and Table 4 shows results of auditory lateralization errors (number of errors) in the training and control groups before and after the auditory lateralization training (percent).
Covariance test showed that in the training group, the mSAAT score in right and left ear, spatial WRS in noise at -90, -60, -30, zero, +30, +60, +90° improved significantly (p value≤ 0.001) and number of lateralization errors at -90, -60, -30, zero, +30, +60, and 90° azimuth decreased significantly (p value≤0.001) after auditory lateralization training.
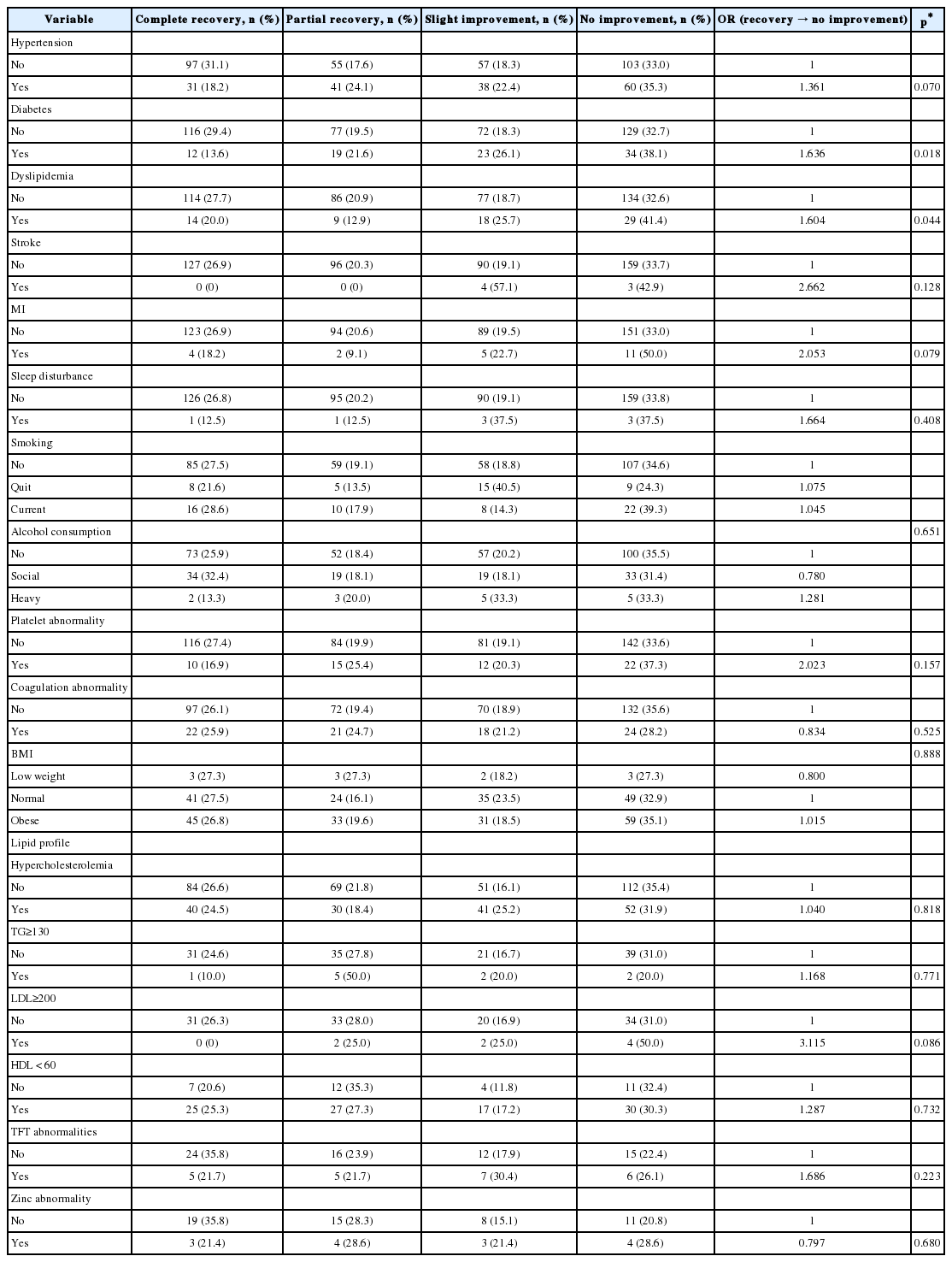
Kolmogorov-Smirnov test showed that mSAAT, spatial WRS in noise and number of auditory lateralization errors at -90, -60, -30, zero, +30, +60, +90° azimuth had non-normal distribution in both training and control groups (p value ≤0.001). Wilcoxon test was used for comparing results within each group. In control group mSAAT score in right ear did not show any significant changes after 2 months (p value= 0.05) but left ear showed significant decline (p value=0.03). In the training group mSAAT score in both ears showed significant improvement after the auditory lateralization training (p value≤0.001) (Table 5). The spatial WRS results showed that in the control group, WRS declined significantly at -30, +30 and +60° azimuth after 2 months. WRS at the remaining positions were unchanged. In the training group, spatial WRS in noise improved significantly at all the positions (p value≤0.001). The number of auditory lateralization errors in the control group did not show any significant change after 2 months (p value was 0.31 at -30° azimuth and 1.00 for other spatial locations). In the training group, there was a significant error reduction at all the locations (p value ≤0.001).
Discussion
Children suspected to (C)APD were selected and based on our hypothesis about importance of spatial abilities in understanding speech in noise/competing signal, they were trained by using an auditory lateralization practice and finally changes in spatial WRS in noise and mSAAT score were tracked. There are few studies about spatial hearing and its relation to speech understanding in noise in children with (C)APD. Studies with similar concepts will be used for discussion.
In this study spatial WRS in noise and mSAAT score in children with (C)APD was more than 2 SDs below normal children and number of lateralization errors were more than 2 SDs higher than normal children before training. These tests can recognize the most common complaint of children with (C)APD which is understanding speech in background noise and in presence of competing signals [827282930]. These results are in agreement with other studies [2327283031323334353637]. This is an important issue because learning at elementary schools are primarily auditory-verbal and classrooms are inherently noisy places [383940414243]. The ability to recognize location of target speech and focusing attention to it when there are other competing signals is critical for understanding speech in everyday situations. Cameron and Dillon created a special and three dimensional speech in noise test under headphone. This test is called listening in spatialized noise-sentence test. They mentioned that a large proportion of children suspected to (C)APD suffer from spatial hearing disorder and it means they are not able to focus on speech that is coming from one direction and suppress simultaneous signals that are coming from other directions. This is why they have more difficulty listening in everyday noisy environments [131428]. Jerger [44] mentioned that main underlying cause for (C)APD (listening problems in noisy environments) can be auditory space representation problems and that spatial hearing problems can give rise to sound source streaming problems [13]. Our study likewise showed that children suspected to (C)APD have more lateralization errors and they have speech understanding difficulties in noise in different spatial directions and when there is a competing signal.
After lateralization training, number of auditory lateralization errors fell down significantly in only training group. Many human and animal studies have shown that appropriate localization/lateralization training can reduce spatial errors in time. Localization/lateralization training has been used in many animal researches, blind humans, and even normal-hearing adults in virtual auditory field researches and in almost all of these researches, spatial training has been found effective [142445464748]. In animals (e.g., owls or ferrets) occluding one ear canal immediately leads to severe increase in localization errors due to changes in binaural cues. After localization experience in this new condition, errors showed significant reduction. This is indicative of high plasticity of auditory localization [46495051]. In blind humans, localization training in format of playing games (e.g., Hoy-Pippi or virtual auditory games) can improve auditory localization skills dramatically in only a few days [5253]. Spatial hearing plasticity is significant during development and still remains plastic in adulthood. Putting a plug inside one ear canal changes spatial cues, but human adults can relearn localization in time [4754]. It seems that relearning is due to reweighting in localization circuits and new spatial maps [54].
After auditory lateralization training mSAAT and spatial WRS score showed significant improvement in only training group. The control group did not show any significant changes. Since we only used auditory lateralization training, these improvements can be attributed to lateralization training. Cameron and Dillon developed and used LiSN & Learn software (NAL, New South Wales, Australia) for remediating spatial processing disorder in children suspected to (C) APD. This software can be used at home and it is a training game. A pair of headphones is used for delivering stimuli. Child has to perceive a target word nested within a sentence that is delivered from zero degree and ignore other competing sentences that are sent from ±90° azimuth. After 120 game sessions, children with SPDs showed 10.9 dB improvements for speech reception thresholds and responses from parents, teachers, and self-reported questionnaires showed positive outcomes [145556].
Finally it should be noted that we used auditory lateralization training for a time period of 6 weeks and showed that auditory lateralization can improve speech understanding in noise significantly. The generalization of this results needs further researches. The authors recommend other studies with higher sample size and auditory lateralization training for more extended time period. Furthermore we recommend follow up evaluations several months after completion of auditory training to see if these results are long term.
Acknowledgments
We thank our colleague Dr. Saeedeh Mehrkian who provided insight and expertise that greatly assisted the research.
Notes
Conflicts of interest: The authors have no financial conflicts of interest.