Current Treatments for Congenital Aural Atresia
Article information
Abstract
Congenital aural atresia is an ear malformation evident at birth, involving various degrees of failed external ear canal development. A true external ear canal is desirable, as devices that replace the canal are inconvenient and expensive. Therefore, an optimal surgical technique is required. Here, we review useful preoperative and operative techniques. Surgical correction is often not the preferred treatment; the hearing outcome is no better than the outcomes afforded by bone-conduction devices, and surgery may be associated with recurrence or complications such as meatal stenosis. Preoperative evaluation and appropriate management are important. Several means of preventing meatal stenosis are discussed in this review.
Introduction
Congenital aural atresia (CAA) is an ear malformation evident at birth, involving various degrees of failed external ear canal (EAC) development. The incidence is one in 10,000 to 20,000 births. Both right ear and male dominance are evident; CAA is typically unilateral. CAA is associated with the Treacher-Collins, Goldenhar, and Crouzon syndromes. The principal symptom of CAA is conductive hearing loss caused by ear canal obstruction and anomalies of the ossicular chain. Surgical repair of the CAA is one of the most challenging procedures and surgical prognoses are variable. The first surgical repair was performed by Kiesselbach in 1883, and this trial ended up with facial palsy [1]. In the 1970s, Jahrsdoerfer introduced key features of a new surgical technique and reported favorable outcomes [2]. In 2019, the International Microtia and Atresia Workgroup (IMAW) published international recommendations for functional ear reconstruction in patients with microtia and CAA in the setting of a multidisciplinary team that agreed on the desired treatment outcomes [3]. Here, we review evidence-based recommendations for the evaluation and management of children with CAA.
Development and Anatomy
It is important to understand the development and anatomy of the external and middle ear before planning a treatment. The external ear is composed of bone and cartilage. The bony EAC lies within the temporal bone and contains very important structures, such as the facial nerve and carotid artery. The ossicles begin to differentiate from week 4 of gestation. The originally fused mass separates to form the malleus and incus by week 8. The Meckel cartilage, head and neck of the malleus, and body and short process of the incus are generated from the first branchial arch. The second branchial arch gives rise to the Reichert cartilage, malleus manubrium, long process of the incus, and stapes superstructure; these structures are completed by week 16 of gestation. The external ear begins to form at week 4 of gestation. By month 3, a primitive auricle is created via fusion of the auricular hillocks (tissue elevations) of His. External auditory canal formation commences with invagination of the first branchial cleft (ectoderm, the external ear) to form a primitive meatus located between the first and second branchial arches. For the next 2 months, invagination of the epithelial plate continues until the first brachial pouch (endoderm, the middle ear) is encountered; the plate then grows laterally. The combined branchial cleft and pouch is termed the meatal plate; this develops into the tympanic membrane. From month 6, medial-to-lateral canalization of the epithelial plate proceeds until the primitive meatus is encountered. Structural development of external ear completes lastly (≤24 week) after serial development of inner and middle ear (≤8 weeks) [4]. At birth, the medial bony tympanic ring and the lateral, membranous cartilaginous region form the external auditory canal. Postnatally, the bony tympanic ring grows into a cylinder (thus increasing in length) until the child is aged 4 to 5 years. The classification of CAA shares common concept with developmental process of middle and external ear. It can be classified into three: stenosis (type A), partial atresia (type B), and total atresia (type C) based on the consensus recommendations published 2019 [3]. Type A refers to narrowing of EAC with slightly deformed or small but intact tympanic membrane. Usually accompanies normally developed ossicular chain with occasional fixation. This type corresponds to Schuknecht type B and Weerda type A. Type B refers to the partial existence of fibrocartilaginous and bony EAC (bony atretic plate) with absent or rudimentary tympanic membrane occasionally not attached to possibly under developed ossicles. This type corresponds to Weerda type B. Type C is completely absent ear canal with various degree of middle ear deformities and corresponds to Schuknecht type C, D and Weerda type C. Earlier the cessation of the development, the extents of deformities are increased which corresponds to type B and C classification. In addition, as the extents of deformity increase, less efficiency of therapeutic approaches is expected.
It is thus apparent that an EAC malformation may or may not be accompanied by middle or inner ear anomalies. Given the serial development of the external and middle ear, an isolated CAA (thus accompanied by normal auricular development) reflects a malformation that developed late in gestation.
Patient Evaluation and Timing of Surgery
Hearing must be evaluated to exclude middle and inner ear anomalies. In patients with bilateral anomalies, objective hearing tests (e.g., auditory brainstem response or auditory steady state response test) should be performed as early as possible. The preoperative hearing threshold affects the prognosis of CAA repair [5]. Preoperative computed tomography (CT) is essential to identify the facial nerve, ossicles, otic capsule, degree of pneumatizaiton, and important vessels, and to allow review of the middle and inner ear components of the Jahrsdoerfer grading system (Table 1). A score greater than 7/10 predicts surgical success. Notably, better preoperative hearing correlates with more hearing improvement after CAA surgery [5]. The choice of operative repair should be made only after middle and inner ear imaging and functional testing. However, it is unclear whether the Jahrsdoerfer grade predicts re-stenosis of the new EAC [6].
In patients with bilateral CAA, rehabilitation of any hearing loss should commence as soon as possible to avoid delays in speech and functional development. Wearable soft- or hard-band bone-conductive devices should be used prior to CAA correction. However, if CAA is unilateral, patient features and parental views should be considered. If possible, surgical correction could be delayed to the age of 6–7 years, especially in cases with auricular anomalies. Rib cartilages which are the autologous materials for auricular reconstruction do not reach the sufficient size until this age. Further information regarding the sequence of the two surgical correction (atresioplasty and auricular reconstruction) and type of graft materials are described later in this review. There are several additional reasons for delay of surgical correction. Patients should be of sufficient age to understand the purpose and goal of surgery, and to meticulously comply with follow-up instructions. Notably, the risk of bony restenosis is reduced with age [7]. Behavioral hearing tests can be performed in older children, and radiation exposure during preoperative CT is associated with fewer risks, relative to exposure in younger children.
Decision-making in the context of revision surgery is more complicated. The indications include re-stenosis, chronic drainage, and recurrence of moderate to severe conductive hearing loss. Surgical correction rates for these conditions range from 69% to 75%; the speech recognition scores show significant improvement [8].
Surgical Methods and Results
The surgical techniques used to treat CAA and conductive hearing loss since the 1970s have been divided into three categories by Cremers, et al. [9]. Fenestration of the lateral semicircular canal for stapedial fixation has been abandoned because of the high risk of complications, including sensorineural hearing loss. Type 3 tympanoplasty connects the newly generated tympanic membrane to the head of the stapes. Today, many surgeons favor canalplasty because of its minimal disturbance of surrounding structures and ease of combination with auricular reconstruction.
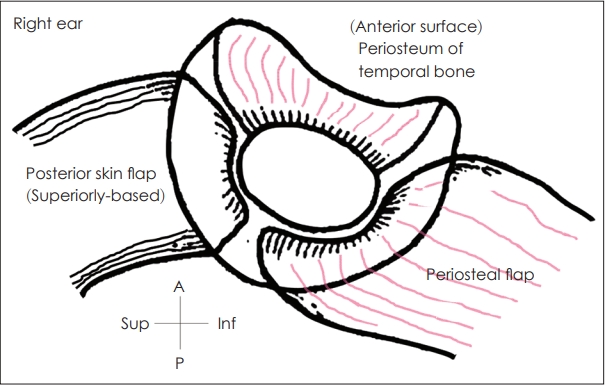
For atresioplasty (canaloplasty) anterior and posterior approaches have been introduced. However, the anterior approach by Jahrsdoerfer is considered the gold standard and adopted in most of cases. Followings are the surgical procedures of atresioplasty with anterior approach. An external auditory canal is created by drilling the atretic plate. A postauricular skin incision, or a Z-plasty incision, allows optimal placement of a rudimentary auricle and subsequent flap positioning (Fig. 1) [10]. Also, a meatal incision is used for formerly reconstructed auricle. The subcutaneous tissue and periosteum are elevated anteriorly to expose the glenoid fossa; an anterior or inferiorbased periosteal flap is then elevated. Drilling commences above the remnant tympanic bone. If there is no bony EAC, drilling may begin above the temporal line immediately posterior to the glenoid fossa. Drilling continues anteriorly and medially until the anterior epitympanum is accessed. Atretic bone (i.e., bony plate that covers the ossicular mass) should be thinned by drilling and removed using curette or elevators to avoid damage to the inner ear. The diameter of the new ear canal should exceed 10 mm. New bony annulus allowing positioning of harvested fascia or sterile and non-immunogenic acellular dermal allograft is then created; this should be medially located at the lateral end of the ossicular mass. Meticulous ossiculoplasty then proceeds, followed by positioning of the harvested fascia and a skin graft of split thickness (0.008-0.011 inch) [11]. To avoid re-stenosis and minimize exposure of the air cavities, it has been suggested that bones lying above the temporomandibular joint should be removed and that the periosteum should serve as the anterior wall of the new ear canal [10]. To minimize the need for a skin graft covering the ear canal, a Z-plasty (a posterior flap) can be used and to cover the exposed mastoid air cells in posterior aspect an inferiorbased periosteal flap can be used (Fig. 2) [10]. Efforts to reduce re-stenosis via placement of a pedicled skin or chondrocutaneous flap have been reported [12-16]. Long-term stenting effectively prevented mental stenosis [17]. The flap-based techniques used to minimize meatal stenosis are summarized in Table 2.
During surgical treatment surgeons must expect to encounter cholesteatoma, because up to 20% of CAA cases are known to accompany cholesteatoma. Surgeons must not underestimate the possibility of facial nerve injury during operation. Aberrant location of facial nerve has to be expected (usually facial nerve is located anteriorly). Surgeons must be careful during the drilling and must preoperatively evaluate the course of facial nerve since it could be the obstacle for canal formation and ossiculoplasty. In addition, surgeons should remind that superficial branches or aberrantly anteriorly located facial nerve exiting to glenoid fossa can be damaged during soft tissue dissection and excessive retraction [7].
Posterior or transmastoid approach can be only considered in cases accompanying extensive cholesteatoma. Transmastoid dissection of the sinodural angle creates a surgical orientation that allows further construction of the new EAC in the absence of any complication [18]. A subset of hearing restoration techniques (lateral semicircular canal fenestration and type 3 tympanoplasty) can be used with this approach.
Ossiculoplasty to treat ossicular chain anomalies (e.g., incudostapedial joint discontinuity, poor lateral chain and/or stapes fixation, and absence of the incus) was required by 6.9-46% of patients who underwent atresiaplasty to reduce the airbone gap and achieve better hearing [19,20]. The type of ossicular reconstruction used may affect the hearing outcome. Placement of a total ossicular replacement prosthesis was associated with worse outcomes than the use of a partial ossicular replacement prosthesis [21]. The audiological result after partial ossicular replacement prosthesis placement was comparable to the result after intact native chain reconstruction. However, Ahn, et al. [21] found that greater prosthesis length was associated with worse hearing outcomes because of the elevated incidence of tympanic membrane lateralization during healing. Because hearing typically deteriorates over time after combined ossiculoplasty/atresiaplasty, the neo-annulus should be placed as medially as possible and prosthesis length should be minimized. A retrospective review of 283 atretic ears treated by means of the anterior approach revealed overall short- and long-term hearing improvements of 30.5 and 22.2 dB, respectively [22]. Similar improvements (approximately 25 dB) have been reported by others [23]. When a transmastoid approach was used to treat 33 ears, the hearing improvement was 23.35 dB, similar to the above figures [18]. Notably, hearing, sound localization, and hearing in noise all demonstrated improvement [24].
CAA is often combined with auricular anomalies. In most patients (more than 90%), atresia or stenosis of the external auditory canal is combined with microtia; robust correlations are evident between the extent of microtia and the frequencies of external and middle ear anomalies [25]. Planned surgical corrections of both anomalies must consider the materials available for auricle reconstruction; commonly used materials include autologous rib cartilage and porous polyethylene (Medpor). When Medpor is used, atresiaplasty should be performed prior to auricle reconstruction [7]. Atresiaplasty following esthetic surgery using Medpor is associated with risks of infection and extrusion of the auricular framework. Skin does not spontaneously cover synthetic material if the framework is exposed. When autologous rib cartilage is used, esthetic surgery should precede atresiaplasty. The plastic surgeon thus has access to a good blood supply and pristine tissue; the risk of scarring is reduced [7]. The IMAW strongly recommends atresiaplasty in combination with (or after) auricular reconstruction using autologous rib cartilage, and before microtia reconstruction using Medpor.
Surgical Repair vs. Bone-Conduction Hearing Aids
Recent technical advances have improved the bone conduction devices used to treat hearing loss. The devices include the Vibrant Soundbridge (MED-EL, Durham, NC, USA), Bone Bridge (MED-EL), Sophono Alpha 1-2 (Medtronic, Jacksonville, FL, USA), and BAHA Attract (Cochlear, Macquarie University, Sydney, Australia). They also include percutaneous osseointegrated bone conduction devices such as the BAHA Connect (Cochlear) and Ponto (Oticon, Copenhagen, Denmark). Such devices must be placed as early as possible in patients with bilateral CAA to prevent delays in speech and language development; this placement is strongly recommended by the IMAW. In patients with unilateral CAA, the need is less clear. A recent study regarding the influence of unilateral CAA on academic performance was inconclusive because of a significant risk of bias [26]. Although the benefits have not been definitively proven, bone conduction devices are useful when unilateral CAA patients are in noisy environments; families should be given the option to evaluate such devices. Most studies have shown that bone conduction devices afforded excellent hearing outcomes. Zernotti, et al. [27] reported 45 dB of improvement in 14 patients who underwent Bone Bridge implantation. When 34 patients received the BAHA (Cochlear) or Bone Bridge (MED-EL) devices, the average hearing improvement was approximately 35 dB [28]. One single-center study directly compared hearing after surgery (n=49) and after BAHA placement (n=19) [29]; the BAHA provided better hearing (≤40 vs. ≤20 dB improvement). Nadaraja, et al. [30] systematically reviewed the hearing outcomes of 107 studies; the average hearing gains afforded by atresiaplasty and osseointegrated bone conduction devices were 24.1 (516 ears) and 38 dB (100 ears), respectively. However, apart from hearing, patient preference and consent to diagnostic imaging must be considered. The quality-of-life and surgical complication rate of atresiaplasty were similar to those of device implantation, although the devices afforded better hearing outcomes [29]. The IMAW recommends that atresiaplasty be performed on certain children older than 6 years of age, with the understanding that subsequent revision surgery may be required. These children should exhibit normal inner ear function and a Jahrsdoerfer score of ≥7.
Conclusion
Atresia repair enhances hearing and improves esthetics. Timing of atresiaplasty is important when such surgery is combined with auricular microtia reconstruction. Bone conduction devices are optional for patients with unilateral CAA, but essential (as early as possible) for patients with bilateral CAA. Treatment options should be discussed with the family in considerable detail.
Acknowledgements
None.
Notes
Conflicts of interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Jeong-Hoon Oh. Investigation: Min Young Lee and Jeong-Hoon Oh. Project administration: Min Young Lee and Jeong-Hoon Oh. Resources: Min Young Lee. Software: Min Young Lee. Supervision: Yang-Sun Cho, Gyu Cheol Han, and Jeong-Hoon Oh. Validation: Yang-Sun Cho, Gyu Cheol Han, and Jeong-Hoon Oh. Visualization: Min Young Lee. Writing—original draft: Min Young Lee. Writing—review & editing: Yang-Sun Cho, Gyu Cheol Han, and Jeong-Hoon Oh. Approval of final manuscript: all authors.