Systematic Review and Meta-Analysis of the Application of Virtual Reality in Hearing Disorders
Article information
Abstract
Background and Objectives
Trendy technologies, such as artificial intelligence, virtual reality (VR), and augmented reality (AR) are being increasingly used for hearing loss, tinnitus, and vestibular disease. Thus, we conducted this systematic review and meta-analysis to identify the possible benefits of the use of VR and AR technologies in patients with hearing loss, tinnitus, and/or vestibular dysfunction, with the aim of suggesting potential applications of these technologies for both researchers and clinicians.
Materials and Methods
Published articles from 1968 to 2022 were gathered from six electronic journal databases. Applying our specified inclusion and/or exclusion criteria, 23 studies were analyzed. As only one article on hearing loss and two articles on tinnitus were found, 20 studies on vestibular dysfunction were only finally included for the meta-analysis. Standardized mean differences (SMDs) were chosen as estimates to compare the studies. A funnel plot and Egger’s regression analysis were used to identify any risk of bias.
Results
High heterogeneity (I2: 83%, τ2: 0.5431, p<0.01) was identified across the studies on vestibular dysfunction. VR-based rehabilitation was significantly effective for individuals with vestibular disease (SMDs: 0.03, 95% confidence interval [CI]: -0.08 to 0.15, p<0.05). A subgroup analysis revealed that only improvement in the subjective questionnaire was meaningful and statistically significant (SMDs: -0.66, 95% CI: -1.10 to -0.22).
Conclusions
VR-based vestibular rehabilitation showed potential for subjective rating measures like Dizziness Handicap Index. The negative effect of aging on vestibular disease was indirectly confirmed. More clinical trials and an evidence-based approach are needed to confirm the implementation of state-of-the-art technology for hearing loss and tinnitus, representative diseases in neurotology.
Introduction
Recently, advanced technologies such as artificial intelligence (AI) and virtual reality (VR) make our lifestyles more convenient and were even boosted by the COVID-19 pandemic by having the advantage of non-face-to-face interactions [1,2]. AI is defined as computer algorithms with the ability to automate cognitive processes [3]. Its concept is extensively smeared in our daily life. That is, no more surprising than the advertisement which we liked on my Google help search specific results. Also, many people are helped by personal assistants [4] like Siri and/or Alexa in iPhone and Bixby in the Galaxy smartphone. These AI techniques consist of various sub-technologies that make it possible to identify the patterns in the big data like providing a graphic-analyzing algorithm for medical imaging analysis [3]. On the other hand, a more sensory-focused simulation technology called VR is also highlighted. VR is defined as an immersed real-time simulation of the user in an interactive environment that mimics reality [5,6]. It has commercialized different games and sports that the whole family can enjoy together by connecting it to a home TV.
Interestingly, these advanced technologies have also influenced the medical fields, especially for otology and/or audiology. For instance, the hearing aid can adjust gain automatically by utilizing machine learning, which is one of the AI subtypes [3,7,8]. The function of volume control and gain initially matched the preferred level of hearing aid users. The machine learning algorithm that is built into hearing aid software provides optimized sound levels in different sound environments. The notion of VR (i.e., sensory interaction including vision, hearing, smell, and touch) is like posturography which performs tridimensional sensory interaction between the visual, vestibular, and somatosensory systems. This method has been usually utilized for VR simulation by using goggles, e.g., the Samsung Gear and Google Cardboard platform [9-11] or the commercially available Computerized Dynamic Posturography device [12] or a video game console, such as Wii® and PlayStation [13]. In addition to these applications, the VR-based technique has been applied for use in tinnitus therapy [1,14], temporal bone surgery [15], and mastoidectomies [16,17].
Regardless, the clinical practice has not yet rapidly adapted to these technological changes. The technologies are being applied only to certain diseases in hearing loss, tinnitus, and vestibular disease. Although some studies have reported the efficacy and/or effectiveness of recent technologies especially for VR in otology by using the systematic review [18-20] and meta-analysis [3,6], most have been either inconclusive or lack enough topic diversity and/or subjective evidence and do not report any statistical significance. By using a systematic review and a meta-analysis approach, we sought to identify the VR technology applied to hearing loss, vestibular dysfunction, and tinnitus, indeed the representative diseases in the fields of otology and audiology. The goal was to analyze its applicability for hearing loss, tinnitus, and vestibular disease and suggest the scope of its potential application for both researchers and clinicians.
Materials and Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [21] and the International Prospective Register of Systematic Reviews (PROSPERO) of Cochrane Collaboration were adjusted to the methodological approach that contained inclusion criteria, an article search strategy, and article selection in the current systematic review and mete-analysis. The protocol of the present study was adopted from a similar methodological approach that is registered in the PROSPERO CRD42011001406.
The specific criteria, a strategy for Participants, Intervention, Control, Outcome measures, and Study design (PICOS), were used for setting up the inclusion criteria. Table 1 displays the PICOS criteria used in this study. Animal studies, general articles (e.g., conference abstracts, proceeding papers, books, and book chapters, and systematic and/or narrative reviews), and articles not written in English were excluded.
Article selection
Six electronic journal databases, Embase, MEDLINE, PubMed, Web of Science, Science Direct, and Cumulative Index to Nursing and Allied Health, were used to search for the articles. Timeframe for the article search and selection was set for January 1968 to February 2022 when articles that initially reported the technology called virtual reality head mounted display [22]. The key terms were ‘hearing loss’ OR ‘dizziness’ OR ‘vertigo’ OR ‘vestibular disease’ OR ‘tinnitus’ AND ‘training’ OR ‘treatment’ OR ‘rehabilitation’ AND ‘virtual reality’ OR ‘augmented reality’ OR ‘metaverse’ OR ‘virtual simulation.’ These terms were combined to minimize the need to filter out any duplicate papers.
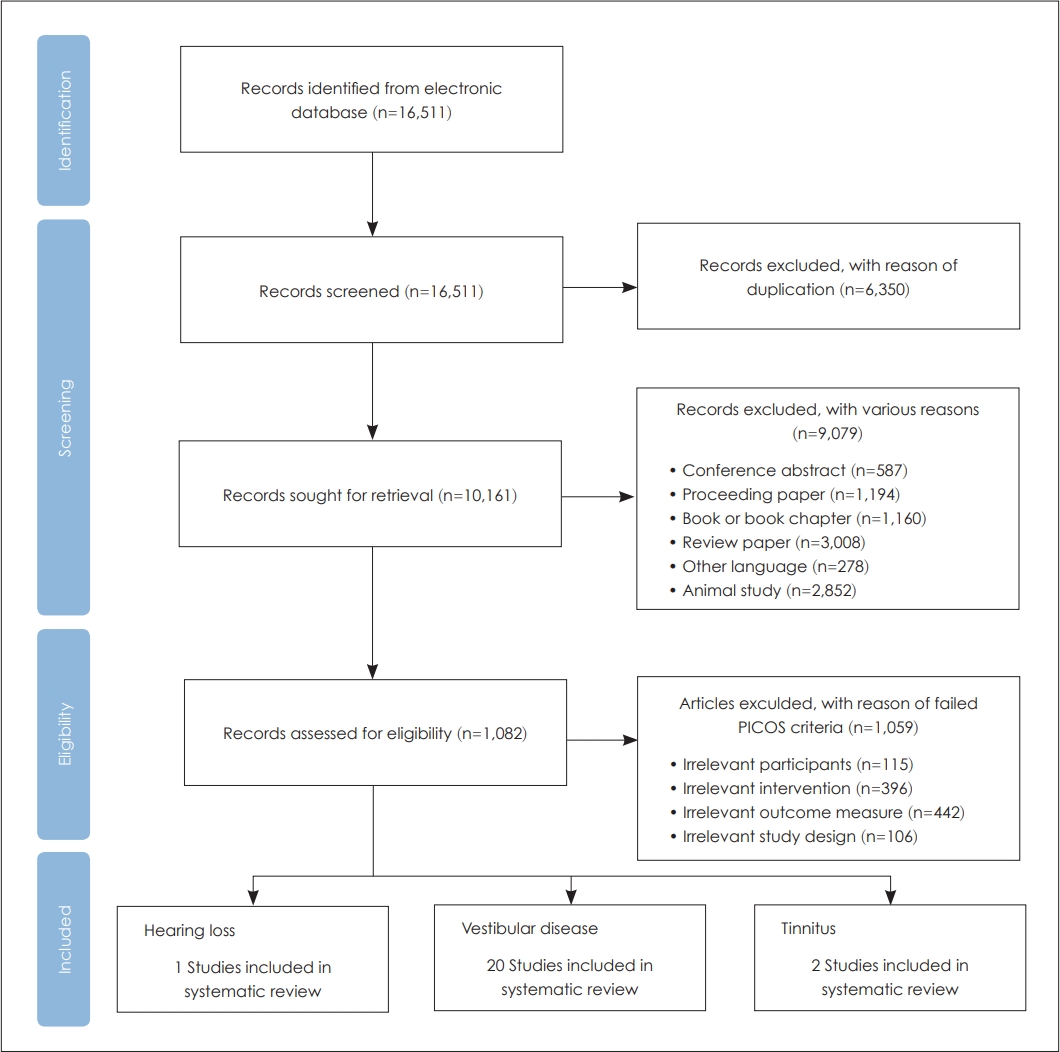
The overall flow of systematic methodology used in the article selection is displayed in Fig. 1. In detail, a total of 16,511 records were searched by using six electronic journal databases. After eliminating 6,350 duplicates, 10,161 records remained. The titles and abstracts of 10,161 records were then screened, resulting in the exclusion of 9,079 records. The full texts of the remaining 1,082 records were then reviewed at the eligibility stage. Finally, only 23 records met the PICOS criteria for this study (Table 1), and they were included in the systematic review and meta-analysis. Throughout all the steps, any disagreement was resolved by consultation with the authors.
Study quality and potential sources of study bias
To evaluate both the study quality and any potential sources of study bias, we used the CAMARADES checklist [23]. This scale assesses the randomization, presence of controls, calculation of sample size, publication after peer review, outcome measures, and statement of potential conflicts of interest (Table 2). Each item was assigned 1 for “yes” or 0 for “no.” The findings of the highest-scoring studies were the most valid.
The data contained in the articles were independently extracted and synthesized into six categories by the authors as 1) participants; 2) intervention; 3) control group; 4) study design; 5) outcome measures; and 6) main findings (Table 3).
Meta-analysis
The R Software (Ver. 4.2.0, R Core Team, R Foundation for Statistical Computing, Vienna, Austria) was used for the meta-analysis. Twenty articles related to vestibular disease were examined for their data synthesis process, especially in terms of their descriptive statistics (mean and standard deviation values in the experimental and control groups). After conducting the data synthesizing, a total of 12 articles were included in the meta-analysis. Standardized mean differences (SMDs) calculated the effect size for the individual study. Then, a summary estimate was examined. The random-effect model was selected to calculate both the effect size and summary estimate. The funnel plot and Egger’s regression test were used to identify any publication bias.
As the confirmation of heterogeneity, the Higgins I2-statistics and Cochran’s Q-test were applied. For the Higgins I2-statistics, the value of I2 was indicated as the percentage of heterogeneity. For example, the interval ranges from 0 to 25%, 25% to 75%, and 75% to 100% of I2 value were implied as having low, middle, and high heterogeneity, respectively [24]. The Q values for the Cochran test indicated the total variance across the dataset of the articles. This test showed a statistical significance at 95% of confidence interval (CI), and heterogeneity across the dataset of articles. The articles were also categorized based on outcome measures, and a subgroup analysis was conducted to compare the area and rate of the Computerized Dynamic Posturography test, the power spectra with low frequency (LF_PS), and a Dizziness Handicap Index (DHI) questionnaire. Using a careful interpretation of the results of meta-analysis, all descriptive values of the meta-analysis in the current study had reversed meaning, which was negative and/or a minus value of outcome measure means a better outcome or being benefited by the intervention and vice versa.
Results
Evaluation of study quality
The study quality evaluated by the CAMARADES checklist showed a mean score of 6.64 (SD: 1.15, range: 4-8). To identify the difference in study quality between studies, a chi-square test was conducted. There were no significant differences between the quality of the studies (χ2=5.8427, df=22, p=0.9998). Table 3 provided characteristics and main findings for all enrolled studies for the participants, the intervention, control group and the outcome of each study [9-14, 25-41].
Overall results of the meta-analysis
Again, the studies related to vestibular disease were included and analyzed using a meta-analysis because of the lack of sample studies in the other fields (i.e., hearing loss and tinnitus). Overall effect size was estimated with the random effect model (Supplementary Fig. 1 in the online-only Data Supplement). The overall estimates showed SMDs of 0.03 (95% CI: -0.08 to 0.15). The heterogeneity related values, like the Higgins I2 and Cochran’s Q estimates (expressed as τ2) demonstrated that there was a high heterogeneity (83% of I2 and 0.5431 of τ2 with p<0.01). In Fig. 2, the funnel plot and Egger’s regression analysis present that there was indeed significant publication bias (t=-7.76, df=183, p<0.0001).
Subgroup analysis
Based on the results of the overall studies, a subgroup analysis was carried out to investigate the actual effects of types of outcome measures (i.e., center of pressure [COP] area and rate, LF_PS, and the score of DHI) (Supplementary Fig. 2 in the online-only Data Supplement). The result of this subgroup analysis was statistically significant (χ2=9.83, df=3, p=0.02). However, the estimate of SMDs for the random effect model was -0.01 (95% CI: -0.17 to 0.14). This result should be interpreted carefully because of the existence of 0 in 95% CI. Even the estimates of the random effect model had a p-value below 0.05, while the existence of 0 in the range of 95% CI was not statistically significant [42].
Given this caution, the result for the COP area (Supplementary Fig. 2A in the online-only Data Supplement) and rate (Supplementary Fig. 2B in the online-only Data Supplement) showed the same value for SMDs of 0.09 (95% CI: -0.15 to 0.33). That is, the experimental group had not benefited from intervention (i.e., conducting vestibular rehabilitation with the VR technique). The result of LF_PS had similar findings. Even the SMDs of LF_PS were -0.01 (Supplementary Fig. 2C in the online-only Data Supplement), and while the experimental group had a better outcome, the statistical significance was not proved (95% CI: -0.49 to 0.47). DHI questionnaire (Supplementary Fig. 2D in the online-only Data Supplement) revealed the only subgroup with statistical significance (SMDs: -0.66, 95% CI: -1.10 to -0.22). This result confirmed that the experimental group had reduced their negative aspects of dizziness with support from the VR techniques.
Discussion
The current study analyzed VR and/or AR technologies being applied to the hearing loss, tinnitus, and vestibular disease. The related studies were all systematically reviewed and analyzed using a meta-analysis approach.
Hearing loss
Unfortunately, the study by Wolter, et al. [25] was only included in the present study. The authors demonstrated that the sound environment and hearing ability influenced the function of balance. They evaluated balance function using a VR simulator-based moving sound environment (i.e., real street setting) and a static sound environment for sensorineural hearing loss with bilateral vestibular loss (SNHL-BVL) children. In addition, the directionality of the sound was added to the sound environment. As expected, the SNHL-BVL children had poorer balance scores than normally developing counterparts in all conditions. With a within-group comparison, the balance score for SNHL-BVL children was not affected by either the VR simulator-based sound environment or directionality. For the effect of hearing ability, SNHL-BVL children had slightly better balance performance when their cochlear implant was activated with variables of the VR simulator-based sound environment and directionality. Results for SNHL-BVL children may have derived from the ability of spatial hearing [25,43]. That is, deteriorated spatial hearing with sensorineural hearing loss made these children insensitive to different sound flows (i.e., dynamic and static sound environments), and this insensitivity led to a similar balance performance in the various VR simulator-based sound environment. However, when their implantation was turned on, the decreased spatial hearing partially supported by the cochlear implant and the balance performance slightly increased. These results demonstrated that the VR technique may be helpful for individuals with hearing loss and balance dysfunction about hearing compensation that occurred when using hearing assistive device. Nevertheless, it is regrettable that only one study exists in this scope. We believe that the current technique will be utilized soon. Also the effect of advanced technologies as a digital therapeutics will be proved by expanding to various population in not only children with hearing loss but also the hearing-impaired adults and elderly.
Tinnitus
In our systematic search, 2 of 23 studies reported on VR related tinnitus management. Although the field of pharmacologic approach and cognitive therapy for tinnitus is rapidly developing, there have been few treatments using VR so far.
Reporting the effect of VR techniques in the field of tinnitus therapy, the studies have been concluded in different ways. First, the study by Bertet, et al. [41] investigated the effect of virtually synthesized tinnitus on the lateralization of tinnitus. They compared three different virtual tinnitus avatars while using hearing thresholds-matched methods and pitch matchinglike mixed tones method. The authors reported that the mixed tones method, by using pure-tone and narrow band noise, was the most preferred for the tinnitus patients (8 of 12 patients). This result was statistically supported by a Kolmogorov-Smirnov test, which statistically compared the visual analogue scale (VAS) of each observation. The ranking of each method revealed that method C (i.e., pitch matching-like mixed tones) had a significantly higher ranking (smaller score of VAS) than method A (p<0.02) and B (p<0.002) which was based on the hearing thresholds curve. Their results demonstrated that the VR-based tinnitus avatar could be applied to tinnitus patients, to some extent.
In the other study, Malinvaud, et al. [14] showed slightly different results even though it had the effect of VR-based therapy on tinnitus patients. The authors compared the VR-based 3D environment with auditory and visual immersion therapy to clinically conventional therapy, such as cognitive behavior therapy (CBT) for subjective tinnitus patients. They measured various outcomes including several questionnaires, e.g., the Subjective Tinnitus Severity Scale, Tinnitus Handicap Questionnaire, Tinnitus Handicap Inventory, Hospital Anxiety-Depression Scale (HAD), and VAS. The results showed that all the outcome measures were not significantly different as the time points (i.e., post treatment, 1-month follow-up, and 3-month follow-up) in both the VR and CBT groups. However, all the outcome measures had significant enhancement from the baseline to 3-month follow-up except for HAD. Rather than quickly concluding the effect of VR on tinnitus treatment through two peer-reviewed papers, thus we suggest that the treatment data should be accumulated and the most efficient VR setting and design for tinnitus patients at clinic and/or at home should be devised.
Vestibular diseases
Although most studies included in the present study were vestibular disease-related, the results of the meta-analysis could show that various outcome measures, such as COP area, COP rate, and LF_PS, were not statistically significant. The effect of the VR technique in the vestibular rehabilitation was not proved for the COP area and rate.
Notably, there was a discrepancy among the studies that reported the effect of VR-based vestibular rehabilitation [10,26-29,32,33,38]. For example, Garcia, et al. [27] demonstrated that those patients with Ménière’s disease (MD) was significantly improved in terms of COP area and DHI score after being treated using VR-based vestibular rehabilitation. However, in other studies [28,29], there was no statistically significant difference between the time points, such as pre- and post- treatment for the benign paroxysmal positional vertigo patients. This discrepancy may have stemmed from aging [6,29]. Obviously, aging directly deteriorates the function of the sensory systems including sensory integration. This decreased sensory integration affected the recurrence of dizziness after treatment [29,38,44]. While the MD patients of Garcia, et al. [27] ranged from young to old adults (age ranged from 19 to 60 years), samples of the other study were middle age to older age adults [26] or adults over 60 years [28,29]. A similar pattern was also observed in other vestibular disease-related outcome measures, such as the rate of COP.
The DHI score revealed that the experimental group actually had a reduced score with VR-based rehabilitation. It was the subjective and self-report measuring method and related to the physical, functional, and emotional aspects of vestibular disease. In other words, the patients with vestibular dysfunction improved their subjective aspects when using VR techniques while being strongly supported in most studies [27,28,32,38]. In short, this result implied that the subjective measures for patients with vestibular disease could be achieved by the implementation of VR [38,45].
Limitations of the study and future directions
Although the purpose mainly was to check how much VR technology was enhanced in hearing loss, tinnitus, and vestibular disease, the present study had several limitations. First, the article search process for the current study had a limitation of not being able to include all the studies related to our purpose. While we exerted a search and select of the articles to avoid this limitation, inevitable variables, such as a lack of explicitness of related topics in the individual article. Similar to the first limitation, the other diseases, i.e., hearing loss and tinnitus, could not lead to a meta-analysis due to the small sample size. We might argue that this limitation emphasizes the diversity of the subfields in otology and audiology. One suspected answer is that the appropriate tools using recent technology are not fully developed yet for individuals with hearing loss.
It is acknowledged that auditory training is necessary and an effective tool for the hearing-impaired regardless of age [46-48], however, conventional and/or traditional auditory training has a disadvantage in terms of time, distance, and cost [46,47]. To overcome the limitation of auditory training, a simulated digital environment called ‘metaverse’ which immerses concept with VR, AR, and the blockchain technique could be considered. This digital world is created for interaction between users like social media and thus may play an important role for the hearing-impaired, including improvement of the social skills and the self-esteem of children.
In conclusion, the present study highlights the recent and advanced VR technologies and their applications to hearing loss, tinnitus, and vestibular disease by systematic review and meta-analysis. Although there was lack of study samples (i.e., two studies for hearing loss and one study for tinnitus), still due to the ongoing development of the technology, VR-based vestibular rehabilitation showed a positive applicability and weaken symptom, especially for subjective rating measures. Also, the negative effect of aging on vestibular disease was indirectly identified. In the future, many more clinical trials and evidence-based approach will be needed to verify the positive implementation of state-of-the-art technology in both hearing loss and tinnitus.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.7874/jao.2022.00234.
Supplementary Fig. 1.
Forest plot of 12 articles included in the meta-analysis.
Supplementary Fig. 2.
Forest plot of subgroup analysis for the outcome measures of center of pressure area (A), center of pressure rate (B), power spectra with low frequency (C), and score of Dizziness Handicap Index (D).
Acknowledgements
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2019S1A5A2A01039904 and NRF-2022S1A5C2A03091539).
Notes
Conflicts of interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: all authors. Data curation: Chanbeom Kwak. Formal analysis: Chanbeom Kwak. Funding acquisition: Junghwa Bahng, Woojae Han. Investigation: Woojae Han, Junghwa Bahng. Visualization: Chanbeom Kwak. Writing—original draft: Chanbeom Kwak. Writing—review & editing: Woojae Han, Junghwa Bahng. Approval of final manuscript: all authors.