Development of Animal Models of Otitis Media
Article information
Abstract
Otitis media is defined as inflammation of the middle ear, including the auditory ossicles and the Eustachian tube. Otitis media is a major health problem in many societies. The causes of otitis media includes infection and anatomic/physiologic, host, and environmental factors. In general, otitis media is a childhood disease, and anatomic and physiologic changes have great effects on its development. Thus, in vitro or human experimental studies of otitis media are difficult. Several experimental animal models have been introduced to investigate the pathogenesis and treatment of otitis media. However, none are ideal. The aim of this review is to provide a brief overview of the current status of animal models of otitis media with effusion, acute otitis media, and cholesteatoma. This review will assist determination of the most appropriate animal models of otitis media.
Introduction
Otitis media is one of the most common childhood diseases and is the most frequent reason for visits to a physician.1) At least four-fifths of children will have experienced one or more episodes of otitis media by the age of 3 years.2) In addition, otitis media affects not only the child, but also the caregiver of the child. It affects hearing and balance, and eventually causes poor language development and poor educational performance.3,4) The financial burden associated with otitis media in children 5 years of age and younger is estimated to be $5 billion annually in the United States.5)
The causes of otitis media include infections and anatomic/physiologic, host, and environmental factors.4) From an anatomical viewpoint, Eustachian tube function is important for preventing the development of effusion in the middle ear. The Eustachian tube contributes to pressure regulation, protection, and clearance of the middle ear cavity.6) Cholesteatomas of the middle ear may also cause otitis media.7) The causes of otitis media commonly overlap with one another. Recently, environmental factors such as smoking and air pollution have been studied in vitro and in vivo.8,9) However, simulating these environmental factors in animal studies is problematic because the appropriate dosages and time frames are unknown.
Experimental otitis media is an active research field in otology. However, studies on humans are difficult in cases of newly developed treatments or drugs. In addition, studies on humans require the results of animal studies according to most research regulations. In vitro research is a good method of drug screening. It requires less time, and dose-response curves are easy to obtain. However, in vitro studies cannot reflect the full range ofanatomical and physiological effects. Several models of otitis media effusion, acute otitis media, and cholesteatoma have been developed (Table 1).10) We herein review the most recently developed animal models and describe our own experience with several of them.
Animal Models of Otitis Media with Effusion
There are two categories of animal models of otitis media. One is Eustachian tube ligation or cauterization using a trans-neck or trans-oral approach,11) and the other is injection of chemical materials through the tympanic membrane.12)
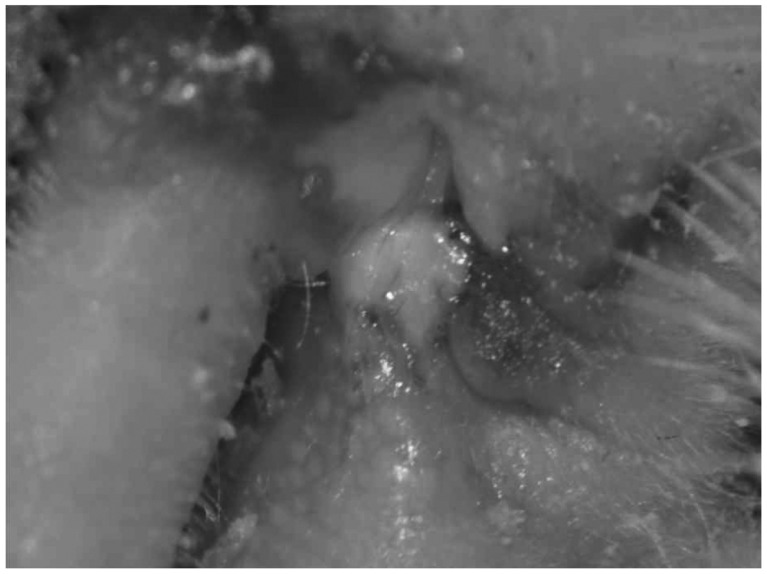
Dysfunction of the Eustachian tube is an important anatomical cause of otitis media with effusion. To simulate this condition, one can block the Eustachian tube by ligation or cauterization. Unfortunately, this is irreversible. The trans-neck approach is an accurate method of blocking the tube. However, it is more time-consuming than the trans-oral approach. Briefly, a rat or other rodent is placed in the supine position after proper intraperitoneal analgesic injection. We prefer tiletamine/zolazepam (Zoletil® 50; Virbac Lab, Carros, France)(0.02 mL/100 mg). A longitudinal midline incision is made at the neck, and the platysma muscle is elevated. After identifying the sternocleidomastoid muscle and belly of the digastric muscle, the facial nerve can be found at distal to the bulla. Arteries that are located medially should be controlled. Through meticulous dissection, the orifice of the Eustachian tube can be seen below the facial nerve and belly of the digastric muscle. Bleeding can be controlled with electrical cauterization (Fig. 1). The orifice of the Eustachian tube can be cauterized, the cartilage portion of the Eustachian tube can be ligated using electrical cautery or nylon, or the tube can be blocked using dental material after the bulla has been exposed.10,11,13) Bacterial pathogens can be added to induce suppurative otitis media.11)
Trans-neck approach to ligation or cauterization of the Eustachian tube for otitis media with effusion. A: Midline incision. B: Elevation of platysma. C: SCM and belly of digastric muscle. D: Exposure of bulla and Eustachian tube orifice. SCM: sternocleidomastoid muscle.
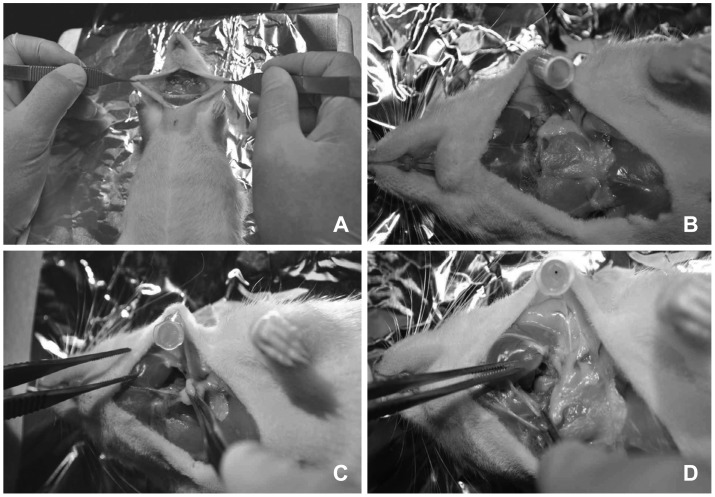
The trans-oral approach is a simpler method of blocking the Eustachian tube.14) However, consistent results are not easy to obtain. After opening the mouth wide using a small blade or mouth gag, an electrical cautery tip is placed on the midline of the soft palate and directed laterally. The orifice of the Eustachian tube is located about 5 mm posterior to the junction of the hard and soft palates (Fig. 2). Severe thermal damage around the Eustachian tube can induce severe bleeding or poor oral intake. Some animals cannot bear this stress. Antibiotics can help to lower the mortality rate. Consistent results are not easy to obtain with this method because of the poor visual field.
Trans-oral approach to ligation or cauterization of the Eustachian tube for otitis media with effusion. A: Electrical cautery of pharyngeal orifice of the Eustachian tube. B: Pharyngeal incision and bulla exposure. C: Three days postoperatively.
When the soft palate is opened with a wide incision, the pharyngeal orifice of the Eustachian tube can be identified under microscopic visualization. This approach provides a better visual field. Trichloroacetic acid (50%) can be used instead of electrical cautery.14)
Injection of chemical materials through the tympanic membrane is easier than the above-described methods. A spinal needle can be used. Histamine solution (0.1 mL) has been shown to induce otitis media with effusion in half of rats 24 h after injection.12)
Spontaneous otitis media with effusion was recently demonstrated in a mouse model of a specific genetic mutation in a G protein-coupled receptor encoded by the Oxgr1 gene.15)
Animal Models of Acute Otitis Media
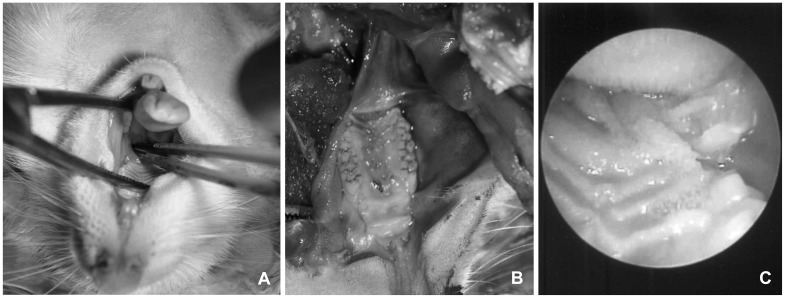
Injection of bacteria through the tympanic membrane is the most common way of inducing acute otitis media in animals (Fig. 3).16) Common causes of infections include Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa are also commonly used in experimental studies. An insufficient inoculum does not induce acute otitis media, and an excess can result in meningitis or sepsis. Thus, bacteria should be diluted to the appropriate levels.
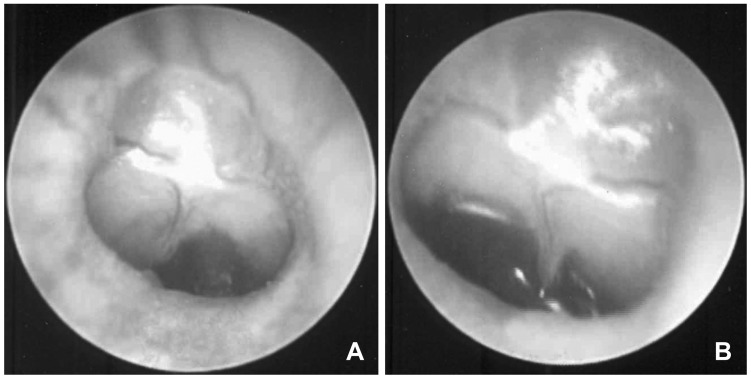
Result 4 days after induction of acute otitis media by inoculation of a bacterial pathogen via the trans-tympanic membrane. A: Right ear. B: Left ear.
Systemic reactivation of otitis media with effusion in a rat model was studied using peptidoglycan-polysaccharide.17) Genetic predisposition has been demonstrated in animal models of middle ear inflammation.18)
Animal Models of Cholesteatoma
Four theories of cholesteatoma development exist: metaplasia, immigration, hyperplasia, and retraction pocket formation. The retraction pocket theory is the most widely used. Animal models of cholesteatoma are usually based one of these theories.
Several categories of animal models of cholesteatoma are available.7) One involves a surgical method, such as external auditory canal ligation, Eustachian tube blocking, or autologous dermal implantation. The other involves the use of chemical materials in conjunction with various delivery methods.
The external auditory canal ligation model is a relatively simple and well-documented method19); however, Mongolian gerbils are required. Mongolian gerbils are useful because cholesteatoma can be easily induced by ligation, and spontaneous cholesteatoma formation is known to occur. Cholesteatoma is induced in 100% of ligated ears; this is a very high success rate in terms of experimental studies of cholesteatoma. Unfortunately, Mongolian gerbils are very expensive in Korea.
Eustachian tube blocking was shown to induce cholesteatoma formation in three-quarters of Mongolian gerbils within 16 weeks.20) Cholesteatoma can be induced by removing the pars flaccida of the tympanic membrane and transplanting a tympanic membrane to the defect of the original tympanic membrane.21)
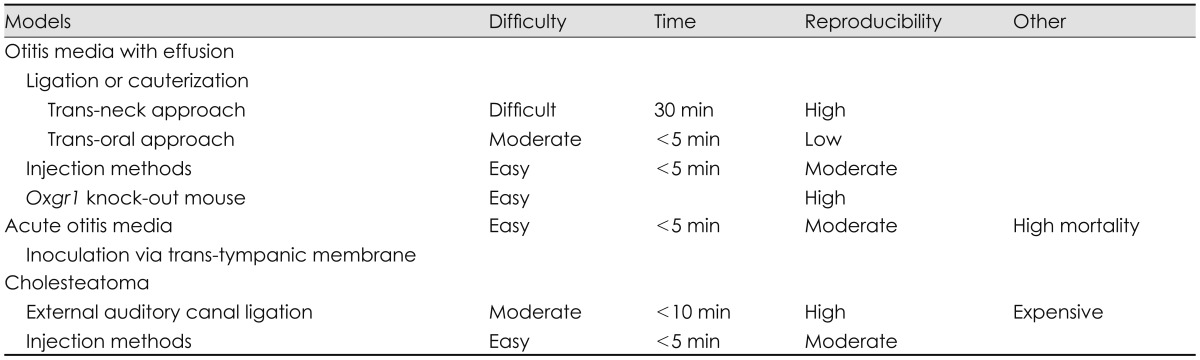
Chemical reagents are usually delivered by injection. Reagents can be injected through the tympanic membrane or bulla. Materials that can induce cholesteatoma include talcum powder, dimethylbenzanthracene, propylene glycol, and latex.22,23) Propylene glycol (concentration, 90%) results in induction of cholesteatoma in most rats (Fig. 4).
Conclusions
Several animal models of otitis media are available. Unfortunately, none are ideal. Researchers who study otitis media should be familiar with the strengths and weak points of each model when selecting the model most appropriate for their purposes. Further physiological animal models of otitis media should be developed.