Effect of Isoflurane on the Hearing in Mice
Article information
Abstract
Background and Objectives
The aim of this study was to investigate the relationship between inhalation anesthetics and hearing in mice.
Materials and Methods
As inhalation anesthetics, isoflurane was used. Auditory brainstem response and distortion product otoacoustic emission were used as measurement of hearing. Mice were divided into 2 groups. 'Isoflurane group' consisted of mice that were anesthetized with an inspired concentration of 2.0 vol% isoflurane with 2 L/min of oxygen (n=10). 'Control group' consisted of mice that were anesthetized with ketamine and xylazine (n=10).
Results
Auditory brainstem response thresholds in mice anesthetized with ketamine and xylazine was not different from those in mice anesthetized with isoflurane. Threshold of DPOAE was higher in mice with isolurane than with ketamine and xylazine. Changes of efferent control may be induced by isoflurane and consequently change the threshold of DPOAE in mice.
Conclusions
These results infer that, there was a change of central nervous system induced by inhalation anesthetics, this change also can be applied to the strategies for prevention of hearing loss.
Introduction
Inhalation anesthetics, such as isoflurane and halothane, can reduce the threshold shift of hearing in noise induced hearing loss model of mice.1,2) Apoptosis of inner ear which was shown in inner ear of noise exposed mice was also reduced in mice with isoflurane anesthetics. Though the mechanism of these protective roles was not known yet, anti-oxidant effect of isoflurane and changes of central nervous system were suggested.3,4)
The protective role in noise-exposed mice was also shown in pentobarbital. Though the protection of hearing against noise was larger in isoflurane than in pentobarbital, modulation of central nervous system should be considered because pentobarbital is known to have little anti-oxidant effect.3,4)
Both of isoflurane and pentobarbital have a muscle relaxant effect. Relaxation of stapes muscle elevated the threshold of stapedial reflex which is protective mechanism of inner ear from noise sound. This means the reduction of protective role from noise in anesthetized mice. However, the reported data showed the contrary results.5-10) Taken together, the study of the relationship between anesthesia and hearing or hearing loss is needed.
Authors aimed to investigate the relationship between anesthetics and hearing in mice. As anesthetics, isoflurane was used. Auditory brainstem response and distortion product otoacoustic emission were used as measurement of hearing.
Materials and Methods
BALB/c mice (4 weeks of age), which have normal Preyer's reflex and a normal hearing threshold, were purchased from Orient Charles River Technology (Seoul, Korea) and were housed in cages and maintained in environmentally-controlled rooms with a 12 h light/dark cycle. Food and water were available ad libitum. All animal experiments were carried out on the approval was obtained from the Animal Care Committee of University of Ulsan College of Medicine. The care and use of the animals reported in this study were in accordance with the guidelines of the Laboratory Animal Unit of Asan Institute for Life Sciences.
Mice were divided into 2 groups. 'Isoflurane group' consisted of mice that were anesthetized with an inspired concentration of 2.0 vol% isoflurane with 2 L/min of oxygen (n=10). 'Control group' consisted of mice that were anesthetized with intramuscular ketamine hydrochloride (30 mg/kg) and intraperitoneal xylazine (2 mg/kg) during measurement of hearing (n=10).
The body temperature of mice was monitored with rectal temperature probe equipped in the continutous monitoring device (Datex-Ohmeda, S/5, Bradford, UK) to rule out the effect of whole body cooling or heating. The mice were maintained at a rectal temperature of 35-37℃ with circulating-water mattress cooling (Blanketrol II, Cincinnati Sub-Zero Co., Cincinnati, OH, USA) and heat lamp.
The hearing level of each mouse was analyzed by measuring the auditory brainstem response (ABR) threshold using an auditory evoked potential workstation (Tucker-Davis Technologies, Alachua, FL, USA). During anesthesia, each ear was stimulated with a probe sealed into the ear canal. To elicit the ABR, click sounds were generated with 1-ms rise/fall times and 2-ms plateaus. To permit recording, subdermal stainless-steel needle electrodes were placed at the vertex and ventrolateral to the left and right ears. ABR waveforms were recorded for 10 ms at a sampling rate of 11.1 kHz using bandpass filter settings of 0.5-3 kHz. Generally, responses from 512 sweeps were averaged to compute a resulting evoked potential. ABR waveforms were recorded in 5-dB steps by decreasing stimulus intensity from the maximum tone-pip level. ABR threshold was determined as the lowest intensity at which a V wave was identified. All waveforms were stored for off-line analysis by an observer blinded to treatment, to obtain average thresholds. Responses were recorded at 4 kHz, 8 kHz, 16 kHz, and 32 kHz. Hearing thresholds are shown as means.
Distortion product otoacoustic emissions (DPOAE) measurements were recorded using an ER10B+ low-noise microphone and a probe housed in the same coupler as the f1 and f2 speakers. The output was passed through an MA3 microphone amplifier and through the RP2.1 A/D converter, where it was sampled at 200 kHz. The magnitude of the 2f1Yf2 distortion product was obtained by fast Fourier transformation of the resulting output using the TDT BioSig software (Tucker-Davis Technologies, Alachua, FL, USA). Distortion product otoacoustic emissions thresholds were calculated at 5.6, 8, 11.3, and 16 kHz.
Stimuli for ABR and DPOAE measurements were calibrated using an ACO calibration kit, EC1 speaker with 0.2-mL coupler, and sigCalRP (Tucker-Davis Technologies).
SPSS version 18 (SPSS Inc., Chicago, IL, USA) was used for data analysis. We used student t-test for analyzing the group difference.
Results
Average ABR thresholds were 18.0±5.9 at 4 kHz, 21.5±4.7 at 8 kHz, 26.5±4.1 at 16 kHz, and 29.0±3.9 at 32 kHz in mice anesthetized with ketamine and xylazine. In mice anesthetized with isoflurane, ABR thresholds were 19.0±5.7, 23.0±3.5, 27.0±3.5, and 31.0±3.2, respectively. ABR thresholds were not different from each other among both groups (Fig. 1). Average thresholds of DPOAE were 29.5±4.4 at 4 kHz, 34.0±3.2 at 8 kHz, 37.5±4.2 at 16 kHz, and 44.0±3.9 at 32 kHz in mice anesthetized with ketamine and xylazine. In mice anesthetized with isoflurane, DPOAE thresholds were 41.0±5.2, 45.5±5.0, 49.5±3.2, and 54.0±5.2, respectively. In mice anesthetized with isoflurane, DPOAE thresholds were higher than those in mice with ketamine and zylazine (p<0.05 in all frequencies)(Fig. 2).
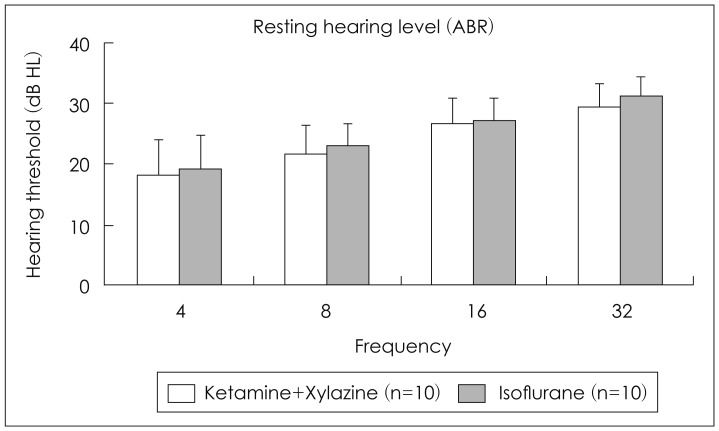
Changes of hearing level measured by ABR according to the anesthesia type. ABR: auditory brainstem response.
Discussion
The effect of anesthesia on ABR is well known. The propofol is known to increase ABR latencies whereas amplitudes are decreased. It has been shown that isofurane is responsible for an increase of ABR latencies whereas it does not modify the amplitudes.11,12)
And propofol is known to decrease both intracranial pressure and cerebral blood flow, but without modifying either cerebral blood flow autoregulation to hemodynamic variation, or vasomotor reaction to PaCO2 variation.13) In contrast, isoflurane has been demonstrated to diminish cerebral blood flow and cerebral blood flow autoregulation.14)
In this study there was no change of ABR threshold in mice with isoflurane, so the function of inner hair cells may not be changed by anesthesia with isoflurane. The results shown in this study could not draw the exact mechanism of the DPOAE change. However, there were couple of reports on the change of DPOAE.
Harel, et al.,15) reported the change of transient evoked otoacoustic emission (TEOAE) by general anesthesia. They showed about 30% increase of threshold in mice with general anesthesia. Likewise, there was 20-35% increase of DPOAE threshold in 4-32 kHz. From these results, isoflurane may have an effect on efferent cochlear control and induced increase of DPAOE threshold.
Ferber-Viart, et al.,16) reported a decrease of TEOAE level by general anesthesia in human study. They proposed the hemodynamic changes in general anesthesia as a responsible cause of TEOAE change. They, however, could not exclude the pharmacological properties of anesthetics. To solve these suggestions, contralateral suppression study is needed. In this study, we didn't perform contralateral suppression study and we only could conclude that the change of cochlear efferent control may be one of the mechanisms of DPOAE change.17-20)
Chung, et al.1) reported that isoflurane and halothane anesthesia could protect noise induced hearing loss in mice. They explained the reduction of the production of reactive oxygen species and antagonistic effect on N-methyl-D-aspartate receptor as possible mechanisms of the protection. They also proposed the protective effect through central processes as one of the mechanisms. Because volatile anesthetics have depressive effect on central function, medial olivocochlear bundle system can be a target of these anesthetics.
The stapedial reflex (SR) is an autonomic reflex that protects the inner ear against very loud noises. The first nuclear connection of the cochlear nerve is at the brain stem and the neurotransmitters involved are not precisely known, but aspartate, glutamate, acetylcholine and noradrenaline (norepinephrine) have been suggested. The same neural mediators have been found in the efferent nuclei of the stapedial reflex arch. The transmitter in the motor endplate is acetylcholine. Because of its neuroanatomical structure, anaesthetic agents, which have a sedative or depressive effect on the central nervous system, may affect the SR.21)
Taken together, changes of DPOAE in mice anesthetized with isoflurane can be explained by the change of efferent control. The exact mechanism of the control needs further study.
Conclusion
If there was a change of central nervous system induced by the anesthetics, this change also can be applied to the strategies for prevention of hearing loss.
Acknowledgments
This work was supported by the Korean Research Foundation grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund; KF-2008-314-E00189).
