|
 |
- Search
| J Audiol Otol > Volume 25(2); 2021 > Article |
|
Abstract
Background and Objectives
The aim of the study was to compare effects of tone-burst (TB) and narrow-band (NB) Claus Elberling (CE)-chirp stimuli on amplitude, latency and interaural asymmetry ratio (IAR) of cervical vestibular evoked myogenic potentials (cVEMP) in healthy individuals.
Subjects and Methods
The study included 50 healthy volunteers. cVEMP procedure was carried out using 500 Hz TB and NB-CE-chirp stimulus (360-720 Hz, up-chirp) in random order. cVEMP were recorded at 100 dB nHL. For each ear and each stimulus, P1 latency, N1 latency and P1N1 amplitude were measured. IAR was also calculated.
Results
Mean age was 26.66┬▒9.48 years. cVEMPŌĆÖs in response to both TB and NB CE-chirp stimuli were obtained in all subjects. No statistically significant difference in P1 latency, N1 latency, and P1N1 amplitude was found between the right and left ears for both TB and NB CE-chirp stimuli (p>0.05). In both sides, P1 and N1 latencies were significantly shorter in NB CE-chirp stimulation compared to TB stimulation (p=0.000). In both sides, no statistically significant difference was found in P1N1 amplitude between two types of stimuli (p>0.05).
Cervical vestibular evoked myogenic potentials (cVEMPs) are inhibitory responses, which originate from the saccule and are directed to the central nervous system by the inferior vestibular nerve following various types of stimulations (sound, vibration, electric) [1,2]. It is a sacculocolic response used in evaluation of the vestibular system [3]. cVEMP is characterized by a biphasic wave and consists of one positive and one negative wave. It is generally named P1N1 (or P13-N23) [4]. In the evaluation of cVEMP response, wave morphology, threshold, latency, amplitude and interaural asymmetry ratio (IAR) are considered [5]. Healthy individuals under 60 years old should have a cVEMP response.
Sensorineural hearing loss does not affect cVEMP while conductive hearing loss does. The IAR is the most common parameter used in the interpretation of the test. It is the ratio of amplitude asymmetry between right and left ears. IAR over 50% is considered definitely abnormal. Prolonged latencies may show a brainstem abnormality. Threshold is the lowest stimulus at which P13-N23 wave is detected. Pathologically low threshold is very important for the diagnosis of Superior Semicircular Canal Dehiscence Syndrome (SSCDS) [6].
Different cVEMPs may be obtained in response to different acoustic stimuli such as click, tone-burst (TB) or chirp. Stimulus type has definite effects on cVEMP response parameters. Several studies across the literature have attempted to describe the best stimulus and recording parameters, however they involved relatively small sample sizes [7].
The chirp is an acoustic stimulus that is designed to compensate the cochlear delay along the cochlear travelling wave. It is so constructed that all frequency parts on the basilar membrane reach maximum depolarization at the same time and generate synchronized firing of the nerve fibers. In detail, high-frequency sound stimuli are presented later than low frequency sound stimuli. The chirp stimulus is a signal in which frequency increases over time (up-chirp) or decreases (down-chirp) [8].
Recent studies have shown that chirp stimulus is effective when recording auditory steady-state responses (ASSR), auditory brainstem response (ABR), and auditory compound action potential. An increased synchronization, larger amplitude waveform and better morphology observed in chirp ABR may allow more reliable interpretation of ABR responses [9].
Information about cVEMP in response to chirp stimulus is very limited in the literature. Also the results are different from each other. In a study conducted in 2013, Claus Elberling (CE)- chirp, click and tone pip stimuli were compared in healty individuals and it was observed that the chirp stimulus had the shortest latency and the largest amplitude [10]. Ûzgür, et al. [11] compared TB, click and chirp stimulation in cVEMP testing in healthy people and they found shorter latencies and lower amplitudes in chirp stimulation. Zakaria, et al. [12] also observed lower amplitude in chirp stimulus in comparison with 500 Hz TB in healthy adults. Interestingly enough, no difference in latency was observed between TB and chirp stimuli. Walther and Cebulla [8] designed a stimulus in the 250-1,000 Hz range (500 Hz) called a band limited chirp stimulus (CWVEMP-chirp) and compared it with a click and short TB stimulus. They thought that the chirp stimulus they used in their study was more effective than other stimuli. Cebulla and Walther [13] used sequential or quasi- simultaneous narrow-band (NB) chirp stimulus at different frequency ranges (middle frequencies of 0.5, 1, 2, and 4 kHz) at 5 normal hearing individuals in 2019. They found that both stimulus were effective in cVEMP response. Finally in 2020, Murofushi, et al. [14] compared CE-chirp level specific cVEMP with 500 and 1,000 Hz TB in 16 patients with vestibular disorders. They found prolonged P1 latency might be an indicator of endolymphatic hydrops.
Bearing some inconsistent results in the relevant literature we aimed to compare effects of TB and NB CE-chirp (upchirp) stimuli on amplitude, latency and IAR of cVEMP in healthy individuals. This type of chirp stimulus was developed by CE and named by giving the first letters of his name and surname. NB-chirps are constructed with octave bandwidth centered at 500, 1,000, 2,000, and 4,000 Hz which increase neural synchronization and also provides frequency specific information.
The study protocol was approved by the Local Ethics Committee on human research (decision no: 18/170, dated: 05. 06.2018). The study included a total of 50 healthy volunteers (21 males, 29 females) with no neurological or otological disease, normal otoscopic examination results and normal pure tone average (PTA). Written informed consent was obtained from all participants.
The study protocol was approved by the local Ethical Committee on Human Research at the Sa─¤l─▒k Bilimleri ├£niversitesi G├╝lhane Traning and Research Hospital, Ankara, Turkey, and was performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki.
Air conduction thresholds at octave intervals from 125 Hz to 8,000 Hz and bone conduction thresholds at octave intervals from 250 Hz to 4,000 Hz were obtained by the same audiologist using the same device (AC40, Interacoustic, Middelfart, Denmark) and the PTA were calculated using 0.5, 1, 2, and 4 kHz.
cVEMP test was performed while the patient was in sitting position in a quiet room. Interacoustic Eclips EP 15 (Interacoustics Eclipse EP15; Assens, Denmark) and insert earphones (Ear Tone ABR 3A; 3M, Minneapolis, MN, USA) were used for the tests. The device was calibrated by a licensed technical personnel according to the International Organization for Standardization 389-6 standards. For cVEMP recording, an active electrode was placed over upper third of the sternocleidomastoid (SCM) muscle, a non-active electrode over the part of the SCM muscle close to the sternum and the ground electrode on the center of the forehead (Ambu┬« NeurolineTM 720; Ambu, Copenhagen, Denmark). Effective contraction of the SCM muscle was obtained by turning the head to the opposite side of the ear being tested. The effective contraction was maintained throughout the test observing visual feedback of the software. As the P13 N23 amplitude is affected by SCM muscle contraction, the subjects were informed regarding visual feedback obtained from the software during the electromyography (EMG) recording to keep the muscle activity at a constant level. Impedance of the electrodes was set to <5 kOhm. 500 Hz TB and 500 Hz NB CE-chirp (360- 720 Hz) stimuli were delivered through earphones in separate recordings. For 500 Hz TB, rise-plateau and fall time were 2-2-2 ms. For 500 Hz NB CE-chirp ranging 360-720 Hz stimuli (up-chirp), stimulus duration was 9 msn. For both stimulus cVEMPŌĆÖs were recorded at 100 dB nHL (113.5 SPL). cVEMP was defined as a biphasic P1N1 (P13-N23) wave with positive polarity at approximately 13 ms (P13) and negative polarity at 23 ms (N23). A consistent potential was reached when the same waveform and latency were obtained in the test, which was repeated twice. EMG signals were amplified (├Ś10,000) and filtered between 10-1200 Hz. Stimulus rate was set to 5.1/s, analysis time to 55 ms and polarity rarefaction. A total of 250 stimuli were averaged. Rectified EMG was taken into account in order to normalize the raw VEMP amplitudes. During the recording, muscle activity was kept between 20-200 ╬╝V root mean square. To eliminate the effect of muscle fatigue in the current study, NB CE-chirp and TB stimuli were delivered in random order.
Data obtained in the study were statistically analysed using SPSS version 22 software (IBM Corp., Armonk, NY, USA). Conformity of the data to a normal distribution was assessed with the Shapiro-Wilk test. When normal distribution was observed, groups were compared using the paired samples t-test. When normal distribution of data was not seen, the two groups were compared with the Wilcoxon signed ranks test. The Wilcoxon signed ranks test was used to compare P1 and N1 latencies between TB and chirp stimuli for both sides (right and left sides). P1N1 amplitude was compared between TB and chirp stimuli using the paired samples t-test for both sides. P1 latency and N1 latency were compared between right and left sides using Wilcoxon signed ranks test while P1N1 amplitude was evaluated with Paired Samples t-test for each stimuli. A value of p<0.05 was considered statistically significant.
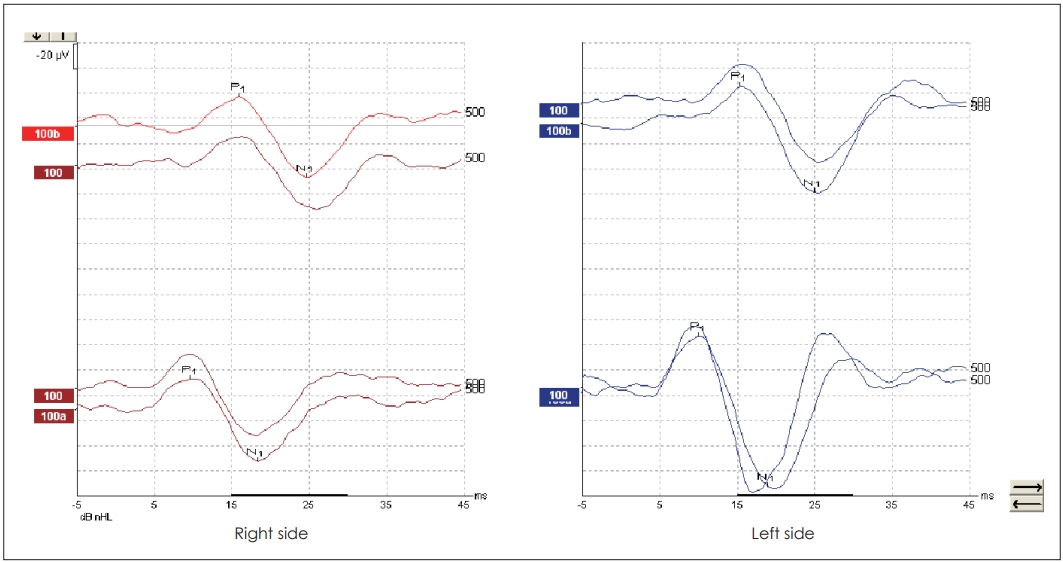
Mean age of the subjects was 26.66┬▒9.48 years ranging from 18 years to 55 years. The PTAs of the healthy individuals was 11.04┬▒3.11 dB (min-max: 7-20 dB). cVEMPs were obtained in all subjects in response to both TB and NB CEchirp stimuli. Characteristic waveforms for both stimuli are shown in Fig. 1. In response to 500 Hz TB stimulus mean P1 latency, N1 latency and P1N1 amplitude were 16.08 ms, 25.30 ms, 55.21 ╬╝V for the right ear and 16.00 ms, 24.70 ms, 51.95 ╬╝V for the left ear, respectively. In response to NB CE-chirp, mean P1 latency, N1 latency and P1N1 amplitude were 10.56 ms, 19.36 ms, and 56.99 ╬╝V for the right ear and 10.36 ms, 19.06 ms, and 52.90 ╬╝V for the left ear, respectively. In comparison between TB and NB CE-chirp stimuli, P1 and N1 latencies were significantly shorter for NB CE-chirp stimulation compared to TB stimulus for the right ears and also for the left ears (p=0.000 for right and left ears, Wilcoxon signed ranks test). On the contrary, no significant difference in P1N1 amplitude was noted for both ears (p=0.466 and p=0.685 for right and left ears respectively, paired samples t-test) (Table 1).
There was no significant difference in comparison of P1 latency, N1 latency and P1N1 amplitude between right and left ears for TB stimulus (p=0.670, 0.109, and 0.2, respectively) and also for chirp stimulus (p=0.304, 0.488, and 0.178, respectively) (Table 1).
Mean IAR values were 0.12┬▒0.10 and 0.15┬▒0.12 for TB stimulus and chirp stimulus respectively.
cVEMP is one of the electrophysiological tests used in the diagnosis of some specific vestibular disorders. The clinical utility of cVEMP in the diagnosis of vestibular pathologies has made it a common method in otology clinics. Even though a 500 Hz TB stimulus was accepted to be a preferred stimulus, an alternative stimulus to produce a robust and steady response can be needed from time to time. The aim of this study was to compare 500 Hz TB stimulus and NB CE-chirp stimulus in order to obtain cVEMP in healthy individuals. In our study, cVEMPs were obtained in all subjects in response to both stimuli. The results showed that while P1 and N1 latencies were significantly shorter for chirp stimulus than for TB stimulus, no difference in P1N1 amplitude was observed between the two types of stimuli. Based on the results in this study, one can propose that NB CE- chirp stimulus is effective in order to produce a robust cVEMP.
cVEMP is a vestibular function test that is used in the evaluation of the saccule and inferior vestibular nerve functions. It is mediated by an ipsilateral pathway that includes the saccular macula, the inferior vestibular nerve, the vestibular nucleus, the vestibulospinal tract, the spinal accessory nerve and the SCM muscle. It has important clinical use in the diagnosis of vestibular diseases such as endolymphatic hydrops, vestibular schwannoma, SSCDS, large vestibular aqueduct syndrome and vestibular neuritis [1].
Stimulus level, stimulus frequency, muscle contraction and electrode location influence cVEMP response such as response rates, latencies and amplitudes. Akin, et al. [15] reported that the widest wave amplitude and lowest thresholds were obtained using 500 and 750 Hz TB stimulus. In addition, semicircular canals are insensitive to 500 Hz stimulus. Therefore, 500 Hz stimulus selectively activates otolitic neurons [16]. In the current study, NB CE-chirp and TB stimuli were delivered at 500 Hz frequency.
VEMP response rate has been reported at 80% to 100% [5]. Ûzgür, et al. [11] found the cVEMP response rate of 93.5% for 500 Hz TB and 89.7% for chirp. On the other side Walther and Cebulla [8] found response rate %90 for both TB and chirp stimulus. In the current study, the response rate obtained from both NB CE-chirp and TB stimuli was 100% which proves utility of NB CE-chirp stimulus as an alternative to TB stimulus. In addition to wave morphology for NB CE-chirp was also quite robust.
Akin, et al. [15] examined the effect of stimulus intensity on latency and reported that a stimulus intensity of 95-100 dB nHL is necessary in order to obtain cVEMPs with no variability on latency. In the light of these finding, we preferred a stimulus intensity of 100 dB nHL in order to observe a difference between TB and NB CE-chirp stimuli at 500 Hz.
In recent years there has been a growing interest in use of chirp stimulus in VEMP in addition to auditory ABR and ASSR tests [8]. Different neural regions along the cochlea are not stimulated at the same time when traditional acoustic stimuli are used, such as click, TB and tone-pip. Chirp stimulus, however, is designed to compensate for time delay in peripheral hearing by increasing the temporal synchroniation between neural structures [17]. Chirp stimulus renders all frequency segments in the cochlea to be stimulated approximately at the same time so that optimum temporal neural synchronicity is obtained which provides extreme effectiveness in the evaluation of cochlear function [17]. This temporal synchronization can be provided by delaying the higher frequencies relative to the lower frequencies of the stimulus [18]. Theoretically, a NB stimulus should be more efficient than TB stimuli while propagating through the middle ear and perilymph fluid on the way to the sacculus. No doubt, middle ear resonance may clip some frequency segments of a given stimulus. In case of middle ear pathology clipping may become greater to the extent of extinguishing response. Shape of TB stimulus appears rather pointed or sharp as opposed to NB chirp stimulus. Rodrigues, et al. [19] recorded ABRs in response to NB 80, 60, 40, and 20 dB nHL of CE-chirp and TB stimuli at 500, 1,000, 2,000, and 4,000 Hz from infants with normal hearing by proposing the hypothesis that more robust wave V amplitudes would be obtained in ABR in response to NB CE-chirp stimulus than TB stimulus. Furthermore, waves at shorter latencies were obtained with NB CE chirp stimulus compared to TB stimulus. However, except at high intensity level (80 dB nHL), greater amplitudes were obtained with NB CE-chirp. However, its direct effect on the vestibular pathway is not yet known.
Chirp is defined as an acoustic stimulation that frequency changes (increasing or decreasing) with time. Many different types of chirp stimulus have been described in literature, such as CE chirp, wide-band chirp, NB chirp, NS chirp, CWC-NB etc. According to Walther and Cebulla [8] when effective VEMP stimulus frequencies are used, chirp stimulus is extremely appropriate for VEMP.
It has been reported that TB stimulus has a longer latency than chirp stimulus [11]. This is explained by the fact that chirp stimulus stimulates wider frequency regions of the cochlea at the same time leading to shorter cochlear stimulation than pure tone stimulation that encompasses rising, plateau and falling time [10]. Another reason for short latency of cVEMP with chirp stimulus is that chirp stimulus stimulates the sacculus more effectively and sensitively [10]. The current study results in shorter latency of P1 and N1 waves with a chirp stimulus which is consistent with the literature.
Previous studies comparing ABR amplitude showed greater amplitudes with chirp stimulus than with other stimuli [19,20]. However, there are some controversial results with regard to cVEMP P1N1 amplitude. For example, Ûzgür, et al. [11] and Zakaria, et al. [12] found higher P1N1 amplitude with TB stimulus compared to chirp stimulus. In contrast, Wang, et al. [10] and Walther and Cebulla [8] reported significantly higher P1N1 amplitude with chirp stimulus. We found that chirp stimulus amplitudes were minimally higher than TB stimulus but there was no significant difference between two stimulus. These differences in the studies may be due to the tuning effect of the otolithic organ and the stimulus and recording parameters used. Also this result can be attributed to the fact that the chirp stimulus stimulates all frequency regions simultaneously, but it was noted that the band-limited chirp stimulus may have the disadvantage of a wider spectral spread than the TB stimulus [21].
Another parameter in the cVEMP test is the IAR. This difference in asymmetry between the ears is especially important in showing the progression of disease [22]. In this study, there was no difference in asymmetry between the ears in both stimuli.
There are a few articles reported the use of chirp stimuli for VEMP measures. Information about cVEMP in response to NB CE-chirp stimuli is also very limited. However, some of these studies have important issues that need to be addressed. In the study of Walther and Cebulla [8], the sample size was too small (n=10) and they used their own stimulus called CW-VEMP-chirp (250-1,000 Hz) stimulus. Later in 2019 the same investigators they used the same chirp stimulus in different frequency ranges (middle frequencies of 0.5, 1, 2, and 4 kHz) for sequential and quasi-simultaneous stimulation of the cervical vestibular organ in 5 normal hearing subjects. However the sample size is also quite small in the study [13]. In this study, 50 healthy individuals were evaluated with CE-NB chirp stimulus. In the study of Ûzgür, et al. [11], the test parameters were lacking. They used 250-4,000 Hz NB chirp stimulus but these frequency range is too large for NB. This makes it difficult to interpret the results correctly. In this study, test parameters are presented in great details. Zakaria, et al. [12] mentioned that they had used chirp stimulus only by referring the study of Ûzgür, et al. [11]. But they did not specify the details of their study. Only Wang, et al. [10] compared CE-NB chirp stimulus with click and ton pip stimulus in 30 healthy subjects in detailed. They found that the latencies are shorter and the amplitudes are larger in the chirp stimulus. Therefore, we think that our study gives more complete findings with a fairly large population.
The main limitation of this study is that it did not include patients with gravitational sensory dysfunction to allow meaningful clinical comparisons between a healthy control grup and a case group.
In conclusion, it is reasonable to conclude that NB chirp stimulus is a good and reliable stimulus alternative to TB stimulus in cVEMP test.
Table┬Ā1.
Cervical vestibular evoked myogenic potentials results based on stimulus type
|
Right |
Left |
p-valueŌĆĪ | |||||
|---|---|---|---|---|---|---|---|
| 500 Hz TB | NB CE-chirp | p-value | 500 Hz TB | NB CE-chirp | p-value | ||
| P1 latency (ms) | 0.000* | 0.000* | >0.05 | ||||
| ŌĆāMean┬▒SD | 16.08┬▒2.18 | 10.56┬▒2.27 | 16.00┬▒2.51 | 10.36┬▒2.39 | |||
| ŌĆāMedian | 15.33 | 9.83 | 15.5 | 9.67 | |||
| ŌĆāMin-max | 12.33-23.33 | 7.33-16.67 | 8.67-21.00 | 6.33-16.33 | |||
| ŌĆāIQR | 2.83 | 3.08 | 3.25 | 3.17 | |||
| N1 latency (ms) | 0.000* | 0.000* | >0.05 | ||||
| ŌĆāMean┬▒SD | 25.30┬▒2.77 | 19.36┬▒2.70 | 24.70┬▒2.78 | 19.06┬▒2.30 | |||
| ŌĆāMedian | 25.16 | 19.00 | 25.00 | 18.67 | |||
| ŌĆāMin-max | 20.33-33.33 | 14.00-24.67 | 16.00-31.00 | 13.33-25.00 | |||
| ŌĆāIQR | 3.84 | 3.83 | 3.92 | 3.51 | |||
| P1N1 amplitude (┬ĄV) | 0.466ŌĆĀ | 0.685ŌĆĀ | >0.05 | ||||
| ŌĆāMean┬▒SD | 55.21┬▒28.54 | 56.99┬▒27.07 | 51.95┬▒25.47 | 52.90┬▒24.49 | |||
| ŌĆāMedian | 49.31 | 51.19 | 51.54 | 50.04 | |||
| ŌĆāMin-max | 14.62-140.80 | 10.30-110.30 | 6.66-138.90 | 14.16-131.30 | |||
| ŌĆāIQR | 39.52 | 46.09 | 35.04 | 34.13 | |||
REFERENCES
1. Fife TD, Colebatch JG, Kerber KA, Brantberg K, Strupp M, Lee H, et al. Practice guideline: cervical and ocular vestibular evoked myogenic potential testing: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 2017;89:2288ŌĆō96.



2. Wu HJ, Shiao AS, Yang YL, Lee GS. Comparison of short tone burst-evoked and click-evoked vestibular myogenic potentials in healthy individuals. J Chin Med Assoc 2007;70:159ŌĆō63.


3. Cheng PW, Huang TW, Young YH. The influence of clicks versus short tone bursts on the vestibular evoked myogenic potentials. Ear Hear 2003;24:195ŌĆō7.


4. Rauch SD. Vestibular evoked myogenic potentials. Curr Opin Otolaryngol Head Neck Surg 2006;14:299ŌĆō304.


5. Isaradisaikul S, Navacharoen N, Hanprasertpong C, Kangsanarak J. Cervical vestibular-evoked myogenic potentials: norms and protocols. Int J Otolaryngol 2012;2012:913515



6. Papathanasiou ES, Murofushi T, Akin FW, Colebatch JG. International guidelines for the clinical application of cervical vestibular evoked myogenic potentials: an expert consensus report. Clin Neurophysiol 2014;125:658ŌĆō66.


7. Meyer N, Vinck B, Heinze B. cVEMPs: a systematic review and meta-analysis. Int J Audiol 2015;54:143ŌĆō51.


8. Walther LE, Cebulla M. Band limited chirp stimulation in vestibular evoked myogenic potentials. Eur Arch Otorhinolaryngol 2016;273:2983ŌĆō91.


9. Bargen GA. Chirp-evoked auditory brainstem response in children: a review. Am J Audiol 2015;24:573ŌĆō83.


10. Wang BC, Liang Y, Liu XL, Zhao J, Liu YL, Li YF, et al. Comparison of chirp versus click and tone pip stimulation for cervical vestibular evoked myogenic potentials. Eur Arch Otorhinolaryngol 2014;271:3139ŌĆō46.


11. ├¢zg├╝r A, ├ćelebi Erdivanl─▒ ├¢, ├¢zergin Co┼¤kun Z, Terzi S, Yi─¤it E, Demirci M, et al. Comparison of tone burst, click and chirp stimulation in vestibular evoked myogenic potential testing in healthy people. J Int Adv Otol 2015;11:33ŌĆō5.


12. Zakaria MN, Zainun Z, Cheu Lih A. Considerations when analyzing vestibular evoked myogenic potential (VEMP) outcomes elicited by chirp stimulus in healthy participants. J Int Adv Otol 2015;11:271ŌĆō2.


13. Cebulla M, Walther LE. Cervical vestibular evoked myogenic potentials via air conduction delivered by either sequentially or quasisimultaneously presented narrow-band chirp stimuli. Int J Audiol 2019;58:174ŌĆō9.


14. Murofushi T, Tsubota M, Tsuda Y, Yoshimura E. Cervical vestibular evoked myogenic potential with chirp sounds. J Vestib Res 2020;30:153ŌĆō8.


15. Akin FW, Murnane OD, Proffitt TM. The effects of click and toneburst stimulus parameters on the vestibular evoked myogenic potential (VEMP). J Am Acad Audiol 2003;14:500ŌĆō9.


16. Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol 2010;121:132ŌĆō44.


17. Elberling C, Call├Ė J, Don M. Evaluating auditory brainstem responses to different chirp stimuli at three levels of stimulation. J Acoust Soc Am 2010;128:215ŌĆō23.



18. Atcherson SR, Moore PC. Are chirps better than clicks and tonebursts for evoking middle latency responses? J Am Acad Audiol 2014;25:576ŌĆō83.


19. Rodrigues GR, Ramos N, Lewis DR. Comparing auditory brainstem responses (ABRs) to toneburst and narrow band CE-chirp in young infants. Int J Pediatr Otorhinolaryngol 2013;77:1555ŌĆō60.


20. Ferm I, Lightfoot G, Stevens J. Comparison of ABR response amplitude, test time, and estimation of hearing threshold using frequency specific chirp and tone pip stimuli in newborns. Int J Audiol 2013;52:419ŌĆō23.