Introduction
Skull base osteomyelitis (SBO) is a fatal disease, especially in people with diabetes or weakened immunity. In these cases, uncontrolled otitis externa spreads to the cranial base through the fissures of Santorini, which leads to osteomyelitis by infiltrating the Haversian system of the compact bone. Symptoms of cranial nerve palsy may develop according to lesion progression, and invasion of the temporal bone may be accompanied by facial nerve palsy. Extensive invasions in cranial nerves IX, X, XI, and XII, which are associated with a poor prognosis, induce hoarseness, dysphagia and aspiration [
1]. A previous study showed that facial nerve palsy is the most frequent symptom (25-60%) of SBO [
2]. Meningitis or brain abscess may also develop in association with SBO. Furthermore, extension of SBO to adjacent tissues can invade the adventitia of the internal carotid artery (ICA), which may result in pseudoaneurysm of the artery with a false lumen [
3]. Pseudoaneurysm is a very rare complication of SBO [
4,
5]. Herein, we report a case of an older patient with an ICA pseudoaneurysm caused by SBO. To the best of our knowledge, this is the first report of such a case in Korea.
Case Report
An 82-year-old female first visited our hospital because of repeated epistaxis and bleeding in the right ear. The patient had a history of diabetes, dementia, and nasopharyngeal cancer, which was in complete remission after the patient underwent chemotherapy and radiotherapy 22 years ago. She also presented with insomnia due to severe otalgia. The patient did not have facial palsy or any symptoms suggestive of cranial nerve palsy. We first suspected malignant otitis externa, SBO, or recurrent nasopharyngeal cancer.
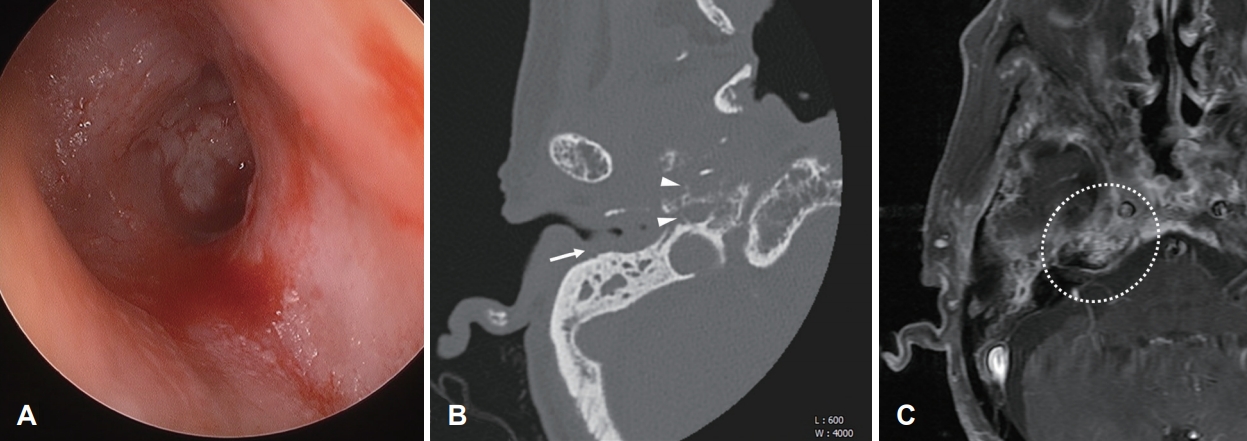
On otoscopic examination, we identified a general narrowing of the right external auditory canal (EAC) and granulation tissue in the middle ear; however, there was no granulation tissue in the EAC. We also observed a bloody and mucous-purulent discharge in the right EAC (
Fig. 1A). Microscopic examination showed detached bone segments in the innermost portion of the right EAC. We performed repeated biopsies of the nasopharynx, along with several cultures of the EAC, temporal computed tomography (CT) and brain magnetic resonance imaging (MRI) (
Fig. 1B,
C). The histopathology of the EAC showed severe bone inflammation; thus, malignancy was excluded.
Escherichia coli, Candida tropicalis, and
Candida albicans were identified in the culture of the EAC. In addition, brain MRI demonstrated inflammation in the skull base with infiltration of the adjacent soft tissue (
Fig. 1C). Based on these findings, a diagnosis of SBO was made, and the patient was prescribed systemic antibiotics (4.5 g piperacillin/tazobactam every 6 hours, 2 g meropenem every 8 hours, and 1 g meropenem every 8 hours for 21, 12, and 3 days, respectively), antifungal agents (100 mg micafungin every 24 hours, 30 mg amphotericin B every 24 hours, and 400 mg fluconazole every 6 hours for 16, 9, and 3 days, respectively) and a strict regimen of insulin (for diabetes).
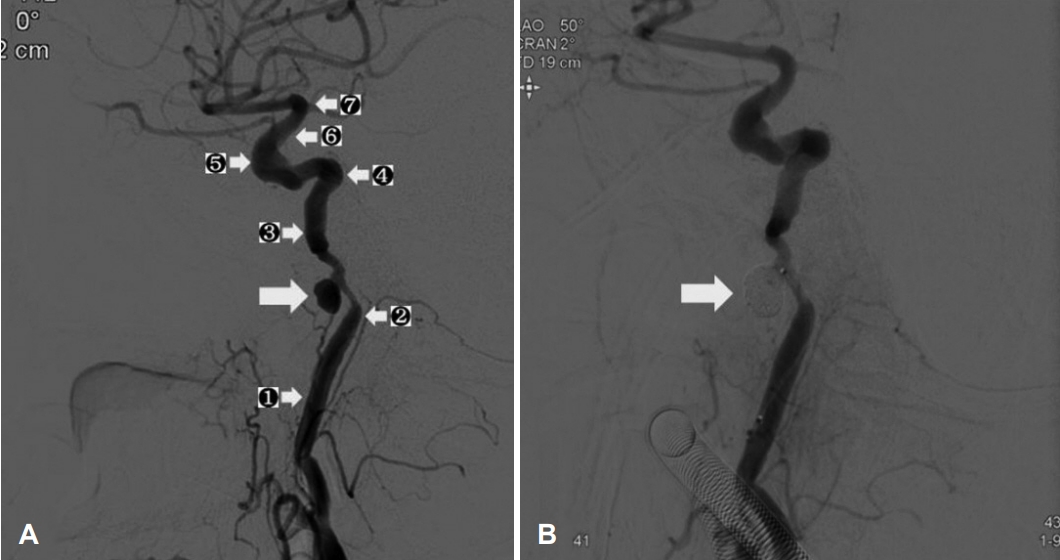
On admission, recurrent massive acute bleeding in the right ear occurred; however, EAC was not involved. This acute, projectile bleeding persisted despite ear packing. We suspected that the bleeding may originate from vessels such as the ICA or the jugular vein, and performed angiography. This revealed a 1.5 cm pseudoaneurysm, which presumably resulted from wall invasion of the petrous segment of the right ICA (
Fig. 2A), and emergency coil embolization was performed (
Fig. 2B). There was no recanalization of the pseudoaneurysm or neurologic symptoms after coil embolization, and the ear bleeding disappeared. No prominent involvement of the zygomatic bone or ethmoid bone was revealed on CT or MRI. Therefore, epistaxis may have been caused by bleeding from the pseudoaneurysm to the nasal cavity through the eustachian tube.
After a week, ear-bleeding recurred, and a repeated angiography was performed. We observed that the previously coiled lesion was enlarged, and performed an additional coil embolization at the pseudoaneurysm base site, which caused recanalization. In addition, multiple stent deployments were performed on the proximal and distal vessels of the pseudoaneurysmfeeding vessel to induce coil stabilization and flow diversion (
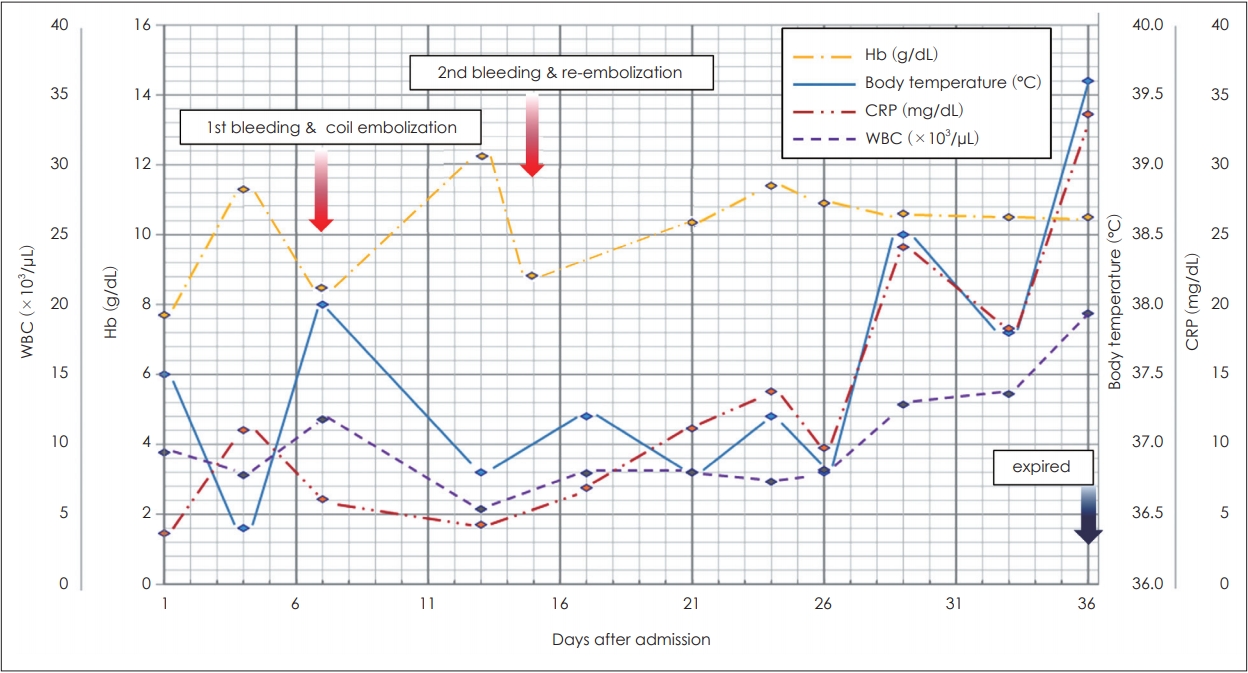
Fig. 3). Subsequently, there was no filling of the pseudoaneurysm or additional bleeding focus. After the first and second hemorrhages, the serum hemoglobin level decreased to 8.5 g/dL and 8.7 g/dL, respectively, and it was maintained above 10 g/dL by transfusion of packed red blood cells each time. There was no further massive bleeding after the last embolization. However, the treatment process was difficult because the patient was old and non-cooperative due to dementia. In addition, the ear dressing was limited because of concerns about rebleeding. Eventually, despite the use of systemic antibiotics, the infection progressed to a septic condition, and the patient died on the 36th day of hospitalization (
Fig. 4).
Discussion
SBO is a devastating disease with a 10-20% mortality rate [
6]. A previous review conducted in 1988 examined 260 cases of SBO, and revealed that 99.2% of infections were caused by
Pseudomonas [
5]. However, recent studies have shown that SBO is caused by
Pseudomonas as well as other pathogens such as
Aspergillus, Proteus, Candida, Staphylococcus aureus, Staphylococcus epidermidis, and
Klebsiella. A previous study showed that SBO was caused by fungal infection in 52% of cases and bacterial infection in 48% of cases.
Pseudomonas aeruginosa caused half of the bacterial SBO [
7]. SBO is defined as the infection and inflammation of the skull base region, including the temporal, sphenoid, and occipital bones. Temporal bone petrous apicitis is defined as an infection or inflammation of the petrous apex and the air cell contained among the temporal bones. These refer to the same disease group and the terms can be used interchangeably depending on where the disease occurs. However, in our patient, the infection and inflammation extended beyond the petrous apex site, and the term SBO was used consistently. In our case, infection with bacteria and fungus was confirmed by culture, and we started combination therapy using antibiotics and antifungal agents.
Pseudoaneurysms of the petrous portion of the ICA are extremely rare. If the inflammation spreads to the ICA vessel wall and adjacent soft tissue from the skull base, it can weaken or erode the adventitia of the vessel and finally make the vesselŌĆÖs false lumen with surrounding soft tissue like a wall [
3]. Previous studies have reported pseudoaneurysms of the petrous portion of the ICA. However, considering the emergency of the lesion and death without definite diagnosis, the prevalence of the lesion may be higher than previously described. If the lesions do not lead to various complications related to the cranial nerve, symptoms or sign for early diagnosis may not have been developed [
4,
5]. In our case, the active bleeding through the eustachian tube originated in the pseudoaneurysm, which can result in ear bleeding and epistaxis rather than neurological problems.
Currently, the therapeutic approach for pseudoaneurysms of the petrous portion of the ICA is open surgical intervention or endovascular occlusion through angiography. While open surgical intervention has an advantage for patients with symptoms, true aneurysms, and larger aneurysms, it is typically performed under general anesthesia [
4]. Considering the high comorbidities or old age of patients with SBO, endovascular intervention is the preferred method for managing the lesion in such patients [
8].
Bleeding of the lesion can be treated using endovascular coil placement or bypass. Endovascular coil placement can be attempted if there is sufficient collateral blood flow because of total occlusion of the ICA balloon during the procedure. Periodic imaging is needed to confirm the changes in the pseudoaneurysm or the presence of collateral blood flow [
9]. Bypass is an appropriate treatment in cases of total obstruction of the ICA during the re-bleeding event. However, in our case, we performed second coiling and multiple stenting, considering the patientŌĆÖs age, massive inflammation, and unstable vital signs.
While some cases of ICA pseudoaneurysm caused by SBO have been reported in other countries, to the best of our knowledge, this is the first report of an ICA pseudoaneurysm caused by SBO in Korea. Based on this case, clinicians should consider a pseudoaneurysm a rare complication of SBO, and be aware of the fact that intractable bleeding may occur. Repetitive epistaxis or ear bleeding, besides symptoms involving the cranial nerves, can be a sign of a pseudoaneurysm, and urgent evaluation of the bleeding is required in such cases. Inpatient treatment must be performed in a hospital equipped with facilities and personnel that allow immediate intervention.