 |
 |
- Search
| J Audiol Otol > Volume 26(4); 2022 > Article |
|
Abstract
Background and Objectives
The aim of this study was to investigate the factors associated with decrease in speech discrimination ability seen in patients with presbycusis and whether aging has a significant effect on the observed decline.
Subjects and Methods
We retrospectively analyzed the medical records of patients aged >40 years who had undergone pure-tone audiometry, speech audiometry, and auditory brainstem response for hearing loss at our hospital from January 2019 to June 2021, and investigated the factors that correlated with speech discrimination score.
Results
We enrolled 103 patients with 120 ears, with a mean age of 65.8┬▒11.9 years. The pure-tone average of the patientsŌĆÖ thresholds at 0.5, 1, 2, and 4 kHz was 37.2┬▒27.7 dB HL, and their mean speech discrimination score was 82.5%┬▒ 22.3%. A correlation analysis revealed a significant negative correlation between the patientsŌĆÖ speech discrimination scores and age. In addition, all variables of pure-tone audiometry and the patientsŌĆÖ auditory brainstem responses were significantly correlated with the speech discrimination scores. The pure-tone average had the strongest negative correlation. On analyzing the significant predictors of lower speech discrimination scores, using a multiple linear regression analysis, pure-tone average and age showed significant results.
Age-related hearing loss (AHL) refers to bilateral symmetrical hearing loss resulting from the aging process [1]. Aging is the primary risk factor for AHL, where the prevalence of hearing loss essentially doubles with each decade of life; e.g., from a prevalence of 13% among Americans aged 40-49 years, to 29% among adults aged 50-59 years, and up to almost 90% among those aged Ōēź80 years [2]. It is characterized by reduced hearing sensitivity and speech understanding in noisy environments, and slowed central processing of acoustic information [3].
There is no basic treatment for AHL, and hearing aids or cochlear implants are used for hearing rehabilitation. One of the most important criteria for prescribing a hearing aid or cochlear implant is the patientŌĆÖs speech discrimination ability; higher speech discrimination ability is associated with greater improvement with the use of a hearing aid.
In patients with AHL, the results of pure-tone audiometry (PTA) generally correlate with the speech discrimination ability. However, the speech discrimination ability may vary among patients with similar pure-tone average. We questioned why speech discrimination ability varied among patients with similar pure-tone average, and tried to find the related factors in this study. For this, we considered the deterioration of the central sound processing ability with aging as the most important factor and investigated changes in the amplitude or latency of the auditory brainstem response (ABR) waveform that indicate the aging-related deterioration of central sound processing and speech discrimination abilities.
We examined whether speech discrimination ability decreased with age, even if the patientŌĆÖs pure-tone average was the same, and what changes in ABR (reflecting the state of the auditory pathway of the brainstem) occur in patients with low speech discrimination ability.
We retrospectively analyzed the medical records of 103 patients aged >40 years who had undergone PTA, speech audiometry, and ABR for hearing loss at our hospital from January 2019 to June 2021. In all, 120 ears were analyzed, excluding those with problems with the middle ear cavity or tympanic membrane, such as chronic otitis media, or with a history of inner ear disease, such as sudden hearing loss or MeniereŌĆÖs disease. Patients with a history of genetic diseases related to hearing loss, autoimmune diseases, or taking anticancer drugs were also excluded.
We investigated the factors that correlated with speech discrimination score. Analyses included patient age, pure-tone average, and amplitude and latency of waves I and V in the ABR.
PTA was performed using a clinical audiometer (GSI61TM; Grason-Stadler Inc., Eden Prairie, MN, USA) calibrated according to the standards of the International Organization for Standardization. Pure-tone air and bone conduction audiograms were analyzed at 0.5, 1, 2, and 4 kHz. Speech discrimination was scored in terms of the percentage of phonetically balanced words that were correctly identified from the HahmŌĆÖs monosyllabic word list [4].
We measured the amplitude and latency of ABR waves I and V at 90 dB normalized hearing level (nHL) in the tested ears using Navigator pro EP (Bio-Logic Systems Corp., Mundelein, IL, USA) and a click sound for stimulation. The output interval was adjusted by 10 dB nHL, and the output range was set at 20-90 dB nHL. The active electrode was placed in the parietal region, the reference electrode was attached to both mastoid processes, and the ground electrode was attached to the forehead. Two-channel electrodes were used for the recordings. The resistance values were adjusted to Ōēż5 k╬®.
After identifying the factors correlated with the speech discrimination score, significant predictors of the score were identified through linear regression analysis.
Pearson correlation analysis was used to investigate the correlation between speech discrimination and other variables, and linear regression analysis was applied to investigate significant predictors of the speech discrimination score. For all statistical analyses, we used SPSS software (ver. 20.0; IBM Corp., Armonk, NY, USA); p values <0.05 were considered statistically significant.
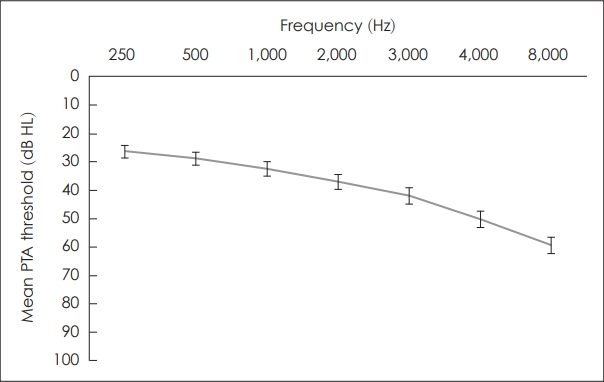
The mean age of the patients was 65.8┬▒11.9 years, the mean pure-tone average was 37.2┬▒27.7 dB HL, and the mean speech discrimination ability was 82.5%┬▒22.3% (Table 1). The average latencies of ABR waves I and V were 1.62┬▒0.27 ms and 5.69┬▒0.44 ms, respectively, and the average amplitudes were 0.10┬▒0.07 ┬ĄV and 0.17┬▒0.09 ┬ĄV, respectively. Because the average age of the patients was high, 21.7% had diabetes, 36.7% had hypertension, and 12.5% had tinnitus. The mean threshold of the PTA for each frequency showed a down-sloping pattern in which the threshold increased as the frequency increased (Fig. 1).
In correlation analyses, there was a significant negative correlation between speech discrimination score and age (Fig. 2). There were no significant differences in the average speech discrimination score according to the presence or absence of chronic disease or tinnitus (data not shown). Comparing speech discrimination score and other hearing test variables, all variables including the PTA threshold for each frequency and speech discrimination score were significantly correlated, and the mean pure-tone average (0.5, 1, 2, and 4 kHz) showed the strongest negative correlation (Table 2). In addition, the speech discrimination score and amplitudes of ABR waves I and V showed a significant positive correlation, whereas the latencies of waves I and V showed a negative correlation. When the significant predictors of speech discrimination score were analyzed through multiple linear regression analysis, the pure-tone average and age showed significant results (Table 3). However, the amplitude or latency of ABR waves I and V were not significant predictors of the speech discrimination score.
The speech discrimination ability of older patients with AHL was significantly reduced by an increase in the pure-tone average and increase in age. There is a consensus that problems with understanding speech in older adults are the result of deteriorated functioning of the cochlea and age-related declines in central auditory processing [5-8]. Our results showed that age and pure-tone average were significant predictors of the speech discrimination score (Table 3). Based on the beta coefficient, the pure-tone average affected speech discrimination scores much more than age. In other words, deterioration of cochlear function had a greater effect on speech discrimination than agerelated decline of central auditory processing.
Studies have reported that a decrease in hearing sensitivity is the major factor influencing the decrease in speech discrimination [9,10]. In a study of Japanese-speaking presbycusis patients, the pure-tone average thresholds at 125 Hz and 8,000 Hz had a negative correlation with speech discrimination for monosyllabic words; the pure-tone average, which showed 50% of speech discrimination, was 76.4 dB HL [10]. Another study of German-speaking patients found that a pure-tone average of 60 dB HL corresponded to a speech discrimination score of 50% [11]. In our study, the pure-tone average threshold at 500 Hz and 4,000 Hz was 82.6 dB HL, which corresponded to 50% of speech discrimination, showing a slightly higher value than the previous results. This is probably due to the different languages and the ages of the subjects included in each study.
However, the most interesting question of this study was whether an increase in age had a significant effect on a decrease in speech discrimination regardless of a decrease in hearing sensitivity. The results showed that increasing age had a significant effect on speech discrimination, probably due to a decrease in the function of central auditory processing with aging (Fig. 2, Table 3). We evaluated the age-related decline in central auditory processing function at the brain stem level by ABR, but there was no significant causal relationship between the change in ABR and speech discrimination in the results of regression analysis.
As shown in Table 2, the ABR parameters showed a significant correlation with speech discrimination scores. In patients with high speech discrimination scores, the ABR amplitude tended to be higher and the latency to be shorter. Previous studies have shown that the ABR amplitude and latency changed significantly with aging, regardless of the deterioration of hearing sensitivity [12]. Many studies have reported that the ABR amplitude decreases and latency increases with aging [13-15].
As mentioned above, aging-related deficits in central auditory processing are associated with reduced speech discrimination. In previous studies, the latency of the cortical auditory evoked potential (CAEP) waveform was significantly increased in patients with low speech discrimination ability [16,17]. Another study found that reduced ABR wave I amplitude, an indicator of cochlear neuronal degeneration, was associated with decreased speech perception ability in noise among older patients [18]. In our study, it was difficult to find a meaningful causal relationship between ABR results and speech discrimination. However, the significant correlation between increasing age and decreasing speech discrimination ability implies that aging negatively affects central auditory processing, leading to a decrease in speech discrimination. Defective central auditory processing is difficult to evaluate with a single hearing test, and should be comprehensively identified through several tests including ABR, CAEP, and cognitive exams.
In conclusion, the speech discrimination ability of older patients with hearing loss significantly decreased with an increase in the pure-tone average and age; of the two, the decrease in hearing sensitivity is the more important factor.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant, funded by the Korean government (grant no. NRF-2020R1C1C1003869).
Fig.┬Ā1.
Average pure-tone hearing threshold by frequency in patients with sensorineural hearing loss. PTA, pure-tone audiometry.
Table┬Ā1.
Demographic and clinical characteristics of the patients
Table┬Ā2.
Correlation analysis between speech discrimination and other hearing test variables
| Variables | Correlation coefficient | p |
|---|---|---|
| Pure-tone audiometry (Hz) | ||
| ŌĆā250 | -0.861 | ’╝£0.001 |
| ŌĆā500 | -0.883 | ’╝£0.001 |
| ŌĆā1,000 | -0.879 | ’╝£0.001 |
| ŌĆā2,000 | -0.821 | ’╝£0.001 |
| ŌĆā3,000 | -0.774 | ’╝£0.001 |
| ŌĆā4,000 | -0.739 | ’╝£0.001 |
| ŌĆā8,000 | -0.700 | ’╝£0.001 |
| Pure-tone average* | -0.868 | ’╝£0.001 |
| Auditory brainstem response | ||
| ŌĆāWave I amplitude | 0.316 | 0.001 |
| ŌĆāWave V amplitude | 0.214 | 0.024 |
| ŌĆāWave I latency | -0.276 | 0.003 |
| ŌĆāWave V latency | -0.377 | ’╝£0.001 |
Table┬Ā3.
Association between speech discrimination score and other factors according to multiple linear regression analysis
|
Multiple linear regression analysis (adjusted r2=0.750, p<0.001) |
|||
|---|---|---|---|
| ╬▓ coefficient | SD | p | |
| Age | -0.210 | 0.102 | 0.042 |
| Pure-tone average* | -0.717 | 0.059 | ’╝£0.001 |
REFERENCES
1. Working Group on Speech Understanding and Aging. Speech understanding and aging. J Acoust Soc Am 1988;83:859ŌĆō95.


2. Flint P, Haughey B, Lund V, Robbins K, Thomas JR, Lesperance M, et al. Cummings otolaryngology: head and neck surgery. 7th ed. Amsterdam: Elsevier;2020.
4. Hahm TY. Articulation function on the Korean speech in patients with hearing impairment. Catholic Med College J 1962;5:31ŌĆō8.
5. Chisolm TH, Willott JF, Lister JJ. The aging auditory system: anatomic and physiologic changes and implications for rehabilitation. Int J Audiol 2003;42 Suppl 2:2S3ŌĆō10.


6. Martin JS, Jerger JF. Some effects of aging on central auditory processing. J Rehabil Res Dev 2005;42(4 Suppl 2):25ŌĆō44.


7. Mazelov├Ī J, Popelar J, Syka J. Auditory function in presbycusis: peripheral vs. central changes. Exp Gerontol 2003;38:87ŌĆō94.


9. Karino S, Usami S, Kumakawa K, Takahashi H, Tono T, Naito Y, et al. Discrimination of Japanese monosyllables in patients with highfrequency hearing loss. Auris Nasus Larynx 2016;43:269ŌĆō80.


10. Maeda Y, Takao S, Sugaya A, Kataoka Y, Kariya S, Tanaka S, et al. Relationship between pure-tone audiogram findings and speech perception among older Japanese persons. Acta Otolaryngol 2018;138:140ŌĆō4.


11. Hoppe U, Hast A, Hocke T. Audiometry-based screening procedure for cochlear implant candidacy. Otol Neurotol 2015;36:1001ŌĆō5.


12. Boettcher FA. Presbyacusis and the auditory brainstem response. J Speech Lang Hear Res 2002;45:1249ŌĆō61.


13. Sand T. BAEP amplitudes and amplitude ratios: relation to click polarity, rate, age and sex. Electroencephalogr Clin Neurophysiol 1991;78:291ŌĆō6.


14. Psatta DM, Matei M. Age-dependent amplitude variation of brainstem auditory evoked potentials. Electroencephalogr Clin Neurophysiol 1988;71:27ŌĆō32.


15. Oku T, Hasegewa M. The influence of aging on auditory brainstem response and electrocochleography in the elderly. ORL J Otorhinolaryngol Relat Spec 1997;59:141ŌĆō6.


16. G├╝rkan S, Durankaya SM, Mutlu B, ─░┼¤ler Y, Uzun Y├¢, Ba┼¤ok├¦u O, et al. Comparison of cortical auditory evoked potential findings in presbycusis with low and high word recognition score. J Am Acad Audiol 2020;31:442ŌĆō8.










