 |
 |
- Search
| J Audiol Otol > Volume 26(4); 2022 > Article |
|
Abstract
Background and Objectives
Chronic suppurative otitis media (CSOM) with or without cholesteatoma is a frequent chronic inflammatory condition in children, which may lead to severe hearing loss that affects speech development. Treatment of recurrent CSOM associated with unserviceable hearing requires a specialized approach with regard to disease eradication and hearing rehabilitation. In this study, we investigated the advantages of subtotal petrosectomy (SP) combined with cochlear implantation (CI) in children with CSOM associated with unserviceable hearing and describe our experience with regard to the efficacy of this method, together with a literature review.
Subjects and Methods
SP with sequential or simultaneous CI was performed in three children (four ears), and postoperative audiometric data were recorded.
Results
The study included two male and one female patient. Mean age at the time of SP was 10.75 years (7ã13 years). Sequential implantation was performed in three ears. Facial nerve palsy occurred after SP in one patient. The latest word recognition scores of Cases 1, 2, and 3 were 80% (at 60 dB), 75% (at 60 dB), and 70% (at 50 dB) and 90% (at 50 dB), respectively.
Chronic suppurative otitis media (CSOM) with or without cholesteatoma is a frequent pediatric chronic inflammatory condition associated with a global incidence of 4.76/1000 individuals [1]. The intermittent or continuous dischargeãcharacteristic of CSOMãmay alter the social life of the children. Moreover, this condition typically leads to hearing loss, influencing speech development [2]. In addition to the conductive hearing loss due to the involvement of the tympanic membrane and the ossicles, sensorineural hearing loss can also occur, especially in cholesteatoma cases [3]. Eventually, children possess a higher risk regarding intra- or extracranial complications [4]. The treatment of CSOM is mainly surgical, and the method is influenced by extent of the disease as well as by preoperative hearing. Canal wall up (CWU) or canal wall down (CWD) tympanoplasty is the most frequently performed technique in cases of CSOM involving the tympanomastoid portion of the temporal bone with serviceable hearing. However, both types can result in disadvantages, such as a wide cavity requiring regular follow-up, limitations in aquatic activities, a worse postoperative hearing for CWD, and a higher recurrence rate regarding CWU [5]. To overcome the drawback of different techniques, bony obliteration tympanoplasty is one of the novel methods regarding cholesteatoma surgery [6]. In such cases, different middle ear prostheses or bone anchored devices can be used to support hearing rehabilitation [7,8].
From another perspective, other possibilities of hearing rehabilitation are worth considering in the cases of CSOM patients with unserviceable hearing. These are usually recurrent cases following multiple surgeries (with profound preoperative hearing loss), frequently with radical cavity or petrous bone cholesteatoma cases. Further hearing loss can be expected in such cases, for example, if labyrinthectomy is performed to fully conquer the disease. These circumstances require special considerations in terms of hearing rehabilitation. For bilateral cases, cochlear implantation (CI) is the only reliable tool. However, in unilateral cases with normal contralateral hearing, contralateral routing of the sound system (CROS) or bone-anchored hearing aids can also be considered, although the superior performance of CI recipients has already been shown in terms of sound localisation and speech comprehension [9].
CI in an infected ear, on the other hand, can lead to biofilm formation or intracranial complications [10]. CI in a chronically diseased ear was contraindicated in the past [11]. Today, CSOM patients are regularly implanted, although the selected surgical methods must be aligned with the following goals: 1) complete elimination of the disease, including the infected mucosa, towards achieving the lowest possible recurrent rate of the disease; and 2) creating a safe, infection-free environment for a successful CI.
Subtotal petrosectomy (SP) coupled with simultaneous or sequential CI is likely an optimal solution for the simultaneous elimination of chronic inflammation and hearing rehabilitation in selected cases [12].
In an SP, a completely closed and dry cavity is created, eliminating all accessible mucosa-containing cells of the tympanomastoidal space. Therefore, the tympanic membrane, the ossicles and the posterior bony wall of the external auditory canal (EAC) are removed, and the sinodural, retrofacial, retrosigmoid, perilabyrinthine, supratubal and pericarotid cells of the temporal bone are exenterated (Fig. 1). At the end of the procedure, the EAC is closed (blind sac closure) along with the Eustachian tube. The created cavity is obliterated using abdominal fat tissue or a pedicled muscle flap. Admittedly, the essential procedure was introduced in the 1950s. Later on, in the 1980s, the precise and extended description including the term ãsubtotal petrosectomyã was published by Coker, et al. [13]. The procedure provides a closed and safe environment for CI.
Considering that only a negligible number of papers have addressed the pediatric engagement of SP and CI, our aim is to present our experiences gained with this combined technique on pediatric patients with CSOM. Nevertheless, to our best knowledge, no paper exists until this date focusing on this surgical solution exclusively in relation to its pediatric aspect.
The medical records of three children were retrospectively reviewed in this study. They all underwent SP with simultaneous or sequential CI between 2018 and 2019 at our department. Demographic data, preoperative audiological and radiological evaluation, complications, and audiological results were analysed.
Preoperative evaluation included microscopic ear examination, subjective audiological examinations, and inner ear MRI. Pure-tone threshold audiometry measurements were performed, completed with speech recognition threshold (SRT) and word recognition score (WRS) using a Danplex DA 45 Audiometer (GN Otometrics, Taastrup, Denmark), in a sound-attenuated chamber. Air conduction measurements regarding pure-tone audiometry were performed at eight different frequencies between 125 Hz and 8,000 Hz, whereas bone conduction was evaluated at six frequencies between 250 Hz and 4,000 Hz. Regarding SRT, 22-digit numbers were presented, and the correctly repeated numbers were tallied (5% awarded to each correct answer). In WRS, monosyllabic words were presented evaluating the correctly repeated answers.
Regarding the audiological tests carried out following the CI, free-field measurements of pure-tone audiometry, SRT, and WRS (1-m distance from the loudspeaker) were performed. The contralateral ear was masked in unilateral cases. Only the evaluated ear was fitted for the bilateral case. The patients were audiologically followed-up with an average of 36 months (range 30-43).
Additionally, 3-T diffusion-weighted (DW) inner ear MRI was performed prior to SP, to confirm the presence of cholesteatoma.
For radiological follow-up, cone-beam computer tomography (CBCT) of the temporal bone was performed postoperatively.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant guidelines on human experimentation and with the Helsinki Declaration of 1975, revised in 2008. The study was approved by the local Ethics Committee (8552/2020). The authors informed the patients and their parents that we publish their data.
Distinctly, the indication regarding SP was based on the following criteria: 1) continuous aural discharge following multiple ear surgeries due to CSOM with cholesteatoma; 2) continuous aural discharge due to CSOM with cholesteatoma without former surgery, but preoperatively confirmed involvement of the otic capsule; and 3) continuous aural discharge following multiple ear surgeries due to CSOM without cholesteatoma, with no serviceable preoperative hearing. The indication for CI was based on the patientsã audiological performance.
All interventions were performed by the last author in general anaesthesia with facial nerve neuromonitoring. The critical surgical steps are detailed here. For the blind sac closure, the anteriorly rotated tragal cartilage was used as the second layer. For the closure of Eustachian tube, the mucosa was elevated first from the bone and pushed back into the tube. The opening was filled with soft tissue covered by Bone Wax (SMI AG; St. Vith, Belgium). For the obliteration of the petrosectomy cavity, abdominal fat was used and a superiorly pedicled musculoperiosteal flap was sutured to the sternocleidomastoid muscle to fix the fat tissue. In the sequential cases, abdominal fat was newly harvested during the CI surgery.
Surgical interventions of four ears were enrolled in this study. The patients were operated between 2018 and 2019. SP and CI were sequentially performed in three ears, and simultaneous CI with SP was carried out in one of these cases. CSOM with cholesteatoma was diagnosed in three ears, and one ear was operated due to CSOM without cholesteatoma. The disease was unilateral in two cases, whereas one patient required bilateral surgery. The mean age at the time of SP was 10.75 years (range 7-13). CI was carried out in the sequential cases at average of 6 months (range 4-12) following SP. Table 1 depicts patient demographic data.
A 7-year-old male patient afflicted with left-sided aural discharge was referred to our department. 3-T DW-MRI previously confirmed cholesteatoma affecting the lateral and anterior semicircular canals (Fig. 2). The preoperative audiogram showed a severe mixed hearing loss on the left side with normal hearing on the right side (Fig. 3A and B). SP was performed with the intraoperative finding of anterior and lateral semicircular canal involvement. Along with the destruction of the incus and stapes superstructure, the matrix was detected in the supra- and infralabyrinthine regions as well. The postoperative audiogram depicted a profound mixed hearing loss (Fig. 3C). WRS on the left side was 70% at 90 dB. CI (Cochlear; Slim Straight electrode, Sydney, Australia) was performed four months following SP. During the procedure, residual matrix was discovered adjacent to the stapes footplate. The postoperative free-field audiogram performed 37 months following CI showed a mild-to-moderate hearing loss (Fig. 3D) with 80% WRS at 60 dB. On the postoperative CBCT scan 37 months after CI, aeration around the Eustachian tube was found without any complains of the patient. No sign of cholesteatoma recurrency was detected.
A 10-year-old male patient underwent 4 ear surgeries on the right side due to cholesteatoma before being presented to our department. Four days before the first intervention, he developed a facial nerve palsy which resolved thereafter. Three another radical mastoidectomies were performed in the next 7 years, mainly due to ear discharge and recurrent cholesteatoma formation. Before the last one, facial palsy occurred again, which resolved after the surgical intervention. On the contralateral side, a ventilation tube was inserted several times.
On our first ear examination (6 months after the last radical mastoidectomy), a radical cavity with massive purulent discharge was revealed. Preoperative DW-MRI confirmed recurrent cholesteatoma located at Prussakãs space, the mastoid cavity directly adjacent to the otic capsule and the tegmen. The audiogram showed severe mixed hearing loss on the right side and a mild mixed hearing loss on the left side (Fig. 4A and B). During the SP procedure, a cholesteatoma matrix was found between the posterior fossa dura and the posterior semicircular canal and between the tegmen and the superior semicircular canal, which could be followed medial to the otic capsule. For complete removal of the disease, labyrinthectomy was performed and the matrix reached the internal auditory canal was removed. Due to the manipulation adjacent to the labyrinthine segment of the facial nerve, a complete facial nerve palsy was observed postoperatively, which recovered to House-Brackmann scale II following oral methylprednisolone treatment. A profound mixed hearing loss was recorded four months following the SP (Fig. 4C) with no word recognition. CI (MED-EL; standard electrode, Innsbruck, Austria) was performed 12 months following the SP. The free-field pure tone audiogram showed a mild-to-moderate hearing loss 30 months after implantation (Fig. 4D). WRS was measured 75% at 60 dB. On the postoperative CBCT scan 30 months after CI, no sign of cholesteatoma recurrency was detected.
A 13-year-old female patient suffering from primary immunodeficiency was presented to our department, with bilateral continuous aural discharge and vertigo. Antrotomy had been performed on the patientãs left side at the age of 4. At the age of 11, CWD tympanoplasty was performed, which was revised 10 months later due to residual cholesteatoma; however, the permanent discharge persisted following the last surgery. DWMRI revealed mastoidal residual disease. The preoperative audiogram showed nearly complete deafness with bone conduction worse than 65 dB above 500 Hz (Fig. 5A). The video head impulse test confirmed a normal bilateral vestibular function. Simultaneous SP with CI (MED-EL; standard electrode) was performed in March 2018. Intraoperatively, a residual cholesteatoma was discovered in the mastoid area. Free-field audiogram 43 months following surgery demonstrated a mild hearing loss with WRS of 70% at 50 dB (Fig. 5B). No sign of cholesteatoma recurrency was detected on the postoperative CBCT scan 30 months postoperatively.
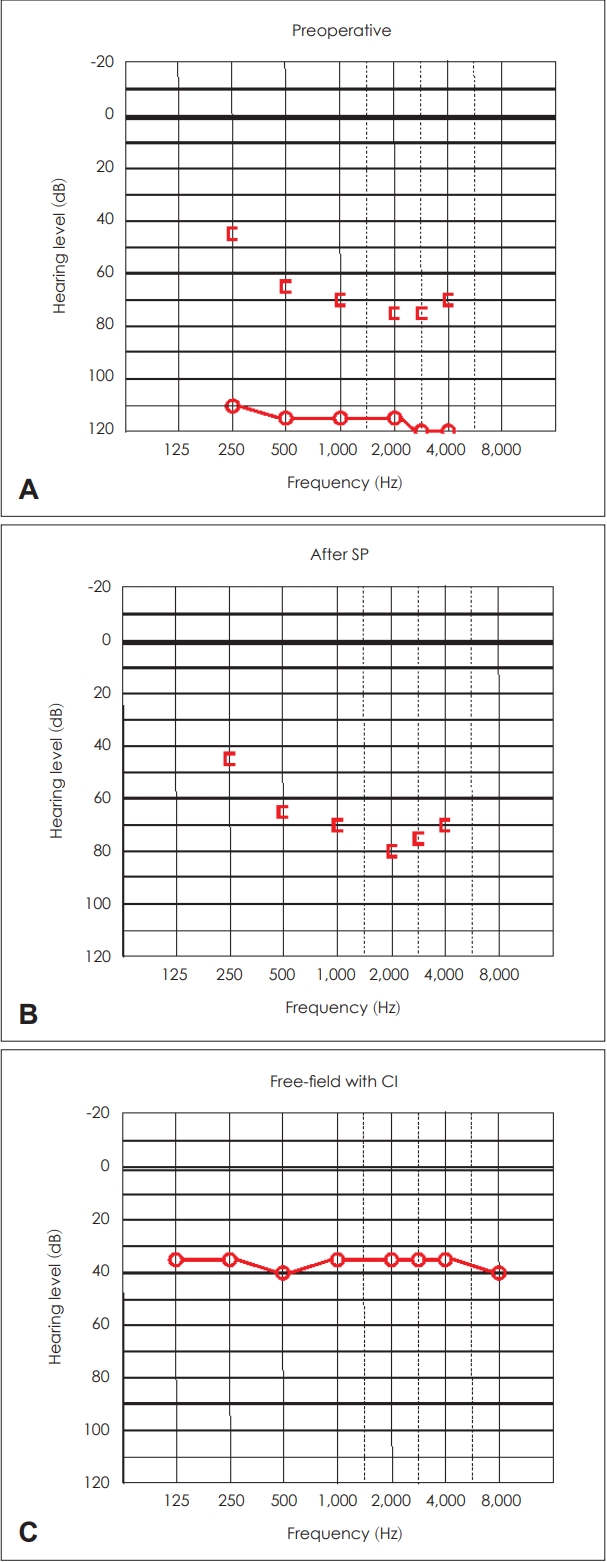
Additionally, in the patientãs right ear, aural discharge with Pseudomonas aeruginosa infection was detected. This ear had previously been operated twice. The preoperative audiogram revealed almost complete deafness, with bone conduction worse than 65 dB above 500 Hz (Fig. 6A). SP was performed in August 2018. During surgery, granulation tissue was found and removed from the tympanic cavity. No deterioration in bone conduction was confirmed on the postoperative audiogram (Fig. 6B). Cochlear implant (MED-EL; standard electrode) was inserted four months following SP through the round window. The free-field audiogram showed a mild hearing loss with WRS of 90% at 50 dB 33 months following implantation (Fig. 6C).
CSOM can lead to hearing loss necessitating rehabilitation. In the case of profound hearing loss, no other reliable solution than CI merits consideration. However, implanting in a potentially infected area always increases the risk of intracranial complications [14]. Moreover, residual disease requires revision, which can alter further electrode functioning.
Regarding safe and stable implantation in CSOM cases, many strategies are recommended. Key questions in these cases include the type of CSOM, the presence of active infection or existing radical cavity, which all have impact on the surgical strategy.
In patients with dry tympanic membrane perforation, myringoplasty followed by CI as a simultaneous or staged procedure is recommended by many authors [15,16]. Himi, et al. [17] suggest second stage implantation following either myringoplasty or CWU tympanoplasty, including removal of the ossicles. Vincenti, et al. [18] reported four patients with inactive CSOM without cholesteatoma. Myringoplasty and CI or one-stage SP and CI were performed in their series, although staged SP and CI were carried out on a patient with active CSOM without cholesteatoma. Even in active cases, Yoo, et al. [19] performed CWU tympanoplasty followed by implantation in case of patients with intact posterior EAC wall. Olgun, et al. [20] reported successful cases with mastoid obliteration together with CI following posterior tympanotomy, or combined with subfacial approach. Grinblat, et al. [21] recently stated that tympanoplasty and second stage CI could only be considered by a rigorous patient selection, and the best outcome is expected to be achieved using SP.
Most authors reported successful cases with the combination of SP and CI in cholesteatoma cases. Staging the procedure is urged in the case of actively discharging ears [15,18,22], although several authors prefer a single-stage procedure even in cases with active discharge [23-25]. Other options such as CWU or CWD tympanoplasties with staged implantation were also reported [17].
Further consideration should be given in cases of longstanding radical cavities. Specifically, without posterior EAC wall, inappropriate covering of the electrode can lead to extrusion requiring revision or explantation. There are various strategies regarding these special circumstances. Vicenti, et al. [18] reported a case of applying open technique in radical cavity, where the implant was protected using pedicled temporalis muscle flap. Interestingly, another patientãs implantation was achieved through a middle fossa approach. Olgun, et al. [20] underlined the importance of strict surgical rules and close follow-up to minimise complications related to CI performed in CSOM patients. They fixed the electrode in the cavity with bone dust and fibrin glue, while mastoid obliteration was achieved using abdominal fat exclusively or combined with cartilage or muscle flap. In several cases, obliteration could be achieved by a combination of subfacial approach or blind sac closure of the EAC. However, recent publications reported the combination of SP and CI as a final solution for patients with severe hearing impairment and associated radical cavity.
In our view, we recommend tympanoplasty and CI only in cases of long-standing dry perforation. From another perspective, in consideration of all active cases (CSOMôÝcholesteatoma) or open cavity cases, no solution other than SP with CI comes into question. Sequential or simultaneous implantation is determined on an individual basis. Regarding unilateral cases, such as Cases 1 and 2, sequential implantation is preferable. However, regarding Case 3, early hearing rehabilitation could be provided by simultaneous SP with CI due to the severe bilateral hearing loss.
Complications of SPs performed due to CSOM are also reported throughout literature. A series of 29 patients with CSOM were treated successfully using SP after 77% of the patients had failed surgeries previously. SP successfully eradicated the disease in all these cases except one, where recurrent cholesteatoma developed six years postoperatively, requiring revision surgery [26]. Szymaéski, et al. [22] demonstrated the safety of SP in 19 CSOM patients who underwent CI. Aeration of the Eustachian tube was revealed based on postoperative CT scan in one case, which soon ceased spontaneously. Free, et al. [27] reported 32 patients, including 4 patients with CSOM and 13 patients with previous CWD surgery, simultaneously implanted with SP. In the case of 1 patient with former CWD tympanoplasty, the middle part of the array extruded through the retroauricular skin, requiring revision.
Our group reported our recent experience gained with SP. The procedure was indicated due to CSOM in 59% of the cases. Intraoperative complications occurred in two cases, including the opening of the lateral semicircular canal with consecutive liquorrhea and wound healing problems. In another case, facial nerve palsy developed immediately following SP due to the proximity of the matrix to the labyrinthine segment of the facial nerve [28].
Although SP and CI for the treatment of CSOM and unserviceable hearing were reported throughout many studies, the number of pediatric cases is considerably limited. Szymaéski, et al. [22] reported a single pediatric case with active discharge, implanted sequentially in six months following SP. Axon, et al. [15] reported a 4-year-old patient with dry perforation and deafness due to leukemia, requiring staged myringoplasty and implantation. Vashishth, et al. [29] reported a 17-year-old patient compelled to explantation following a single-stage SP and CI, due to cavity infection and a retroauricular fistule. Re-implantation was successfully carried out four months following the explantation. SP with CI was carried out in two pediatric cases afflicted with CSOM in the study of Grinblat, et al. [23]. In other studies, pediatric cases are only tangentially mentioned in a heterogeneous patient population [20,27]. Reviewing literature comprehensively, we can point out that our case series is the first study representing exclusively pediatric patients with CSOM undergoing SP and CI.
Another aspect to be highlighted is the radiological followup of the cholesteatoma with non-echoplanar DW-MRI [30]. Though this method is widely accepted and used, due to the shadow effect, proper post-implantation follow-up is challenging. Therefore, CBCT was performed for all the children searching for indirect signs of recurrent cholesteatoma like bony wall involvement. Three years after the CI, no signs of recurrency could be found.
Finally, it should be noted, that in the unilateral cases (Cases 1 and 2), deterioration of the bone conduction after SP could be measured. We believe that this could be a result of the manipulation around the otic capsule, but we do not consider this outcome as a failure. In Case 1, the preoperative MRI already confirmed the involvement of the otic capsule and in Case 2, labyrinthectomy should have been performed for the complete eradication of the cholesteatoma, therefore a further hearing loss could be expected.
Besides it is also to be stated, that for Cases 1 and 2 various solutions for hearing rehabilitation were considered. As we prefer providing true binaural hearing, especially for pediatric patients, we decided to offer CI instead of CROS or bone-anchored devices after a detailed consultation with the parents. Moreover, the patient of Case 2 was already out of indication for a bone-anchored device, because the air conduction threshold of the contralateral ear appeared to be worse than 20 dB. Theoretically, round window vibroplasty could have been also an option in Case 1. The bone conduction threshold after SP was 45 dB at 500 Hz, and 65 dB at 2,000 Hz, equalling the maximal bone conduction threshold declared by the manufacturer. However, further deterioration of bone conduction due the coupling could not be excluded [31].
The treatment of recurrent CSOM with unserviceable hearing requires a special consideration in terms of both the eradication of the disease and hearing rehabilitation. In our study, we performed SP with simultaneous or sequential CI in a pediatric population. Our view is that the closure of the EAC after SP assures a normal social life for the affected children, without any limitation. On the other hand, the obliterated and mucosa-free middle ear cavity serves as a safe environment for CI, providing the best outcome for a severe or profound hearing loss. Although a small number of individuals were enrolled in our study, the long-term complication-free follow-up period and the favourable hearing results suggest that this technique is a safe, reliable, and effective method for pediatric patients with CSOM and unserviceable hearing.
Notes
Author Contributions
Conceptualization: Peter Bako, Imre Gerlinger. Data curation: Peter Bako, Marton Kovacs. Investigation: Peter Bako, Marton Kovacs, Adrienn Nemeth. Methodology: Peter Bako, Marton Kovacs, Adrienn Nemeth. Project administration: Janos Uzsaly, Greta Bodzai. Supervision: Istvan Szanyi, Imre Gerlinger. Visualization: Arnold Toth, Marton Kovacs. Writingãoriginal draft: Peter Bako, Andras Burian. Writingãreview & editing: Andras Burian, Istvan Szanyi, Imre Gerlinger. Approval of final manuscript: all authors.
Fig.ô 1.
Schematic view of subtotal petrosectomy cavity with cochlear implantation. During the procedure, the following structures are exposed leaving only a thin covering bone shell: sigmoid sinus (SS), facial nerve (FN), labyrinth (La), promontory (P), Eustachian tube (ET) and internal carotid artery (IC). All mucosa lining cells are removed: sinodural (SD) retrofacial (RF), retrosigmoid (RS), retrolabyrinthine (RL), supralabyrinthine (SL), infralabyrinthine (IL), supratubal (ST) and pericarotid cells (PC).

Fig.ô 2.
Preoperative MR images of Case 1. A: Axial T2-weighted (SPACE) image shows the involvement of the left lateral semicircular canal (red arrow). B: Axial diffusion-weighted (HASTE) image of the corresponding slice confirms the presence of cholesteatoma. C: Axial T2-weighted (SPACE) image shows the involvement of the left anterior semicircular canal (red arrow). D: Axial diffusion-weighted (HASTE) image of the corresponding slice confirms the presence of cholesteatoma. SPACE, sampling perfection with application optimized contrasts using different flip angle evolution; HASTE, half-Fourier acquisition single-shot turbo spin echo.
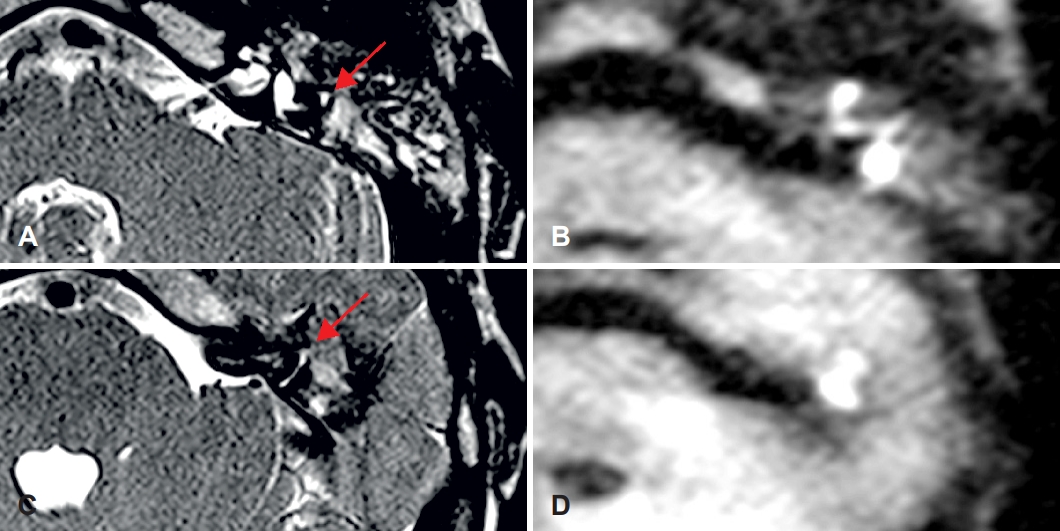
Fig.ô 3.
Pure tone audiograms of Case 1. A: Preoperative pure tone audiogram of the left ear showing a severe mixed hearing loss. B: Normal hearing of the contralateral ear. C: Audiogram of the left ear after subtotal petrosectomy (SP) demonstrating a profound mixed hearing loss. D: Postoperative free-field audiogram 37 months following cochlear implantation (CI) showing a mild-to-moderate hearing loss.
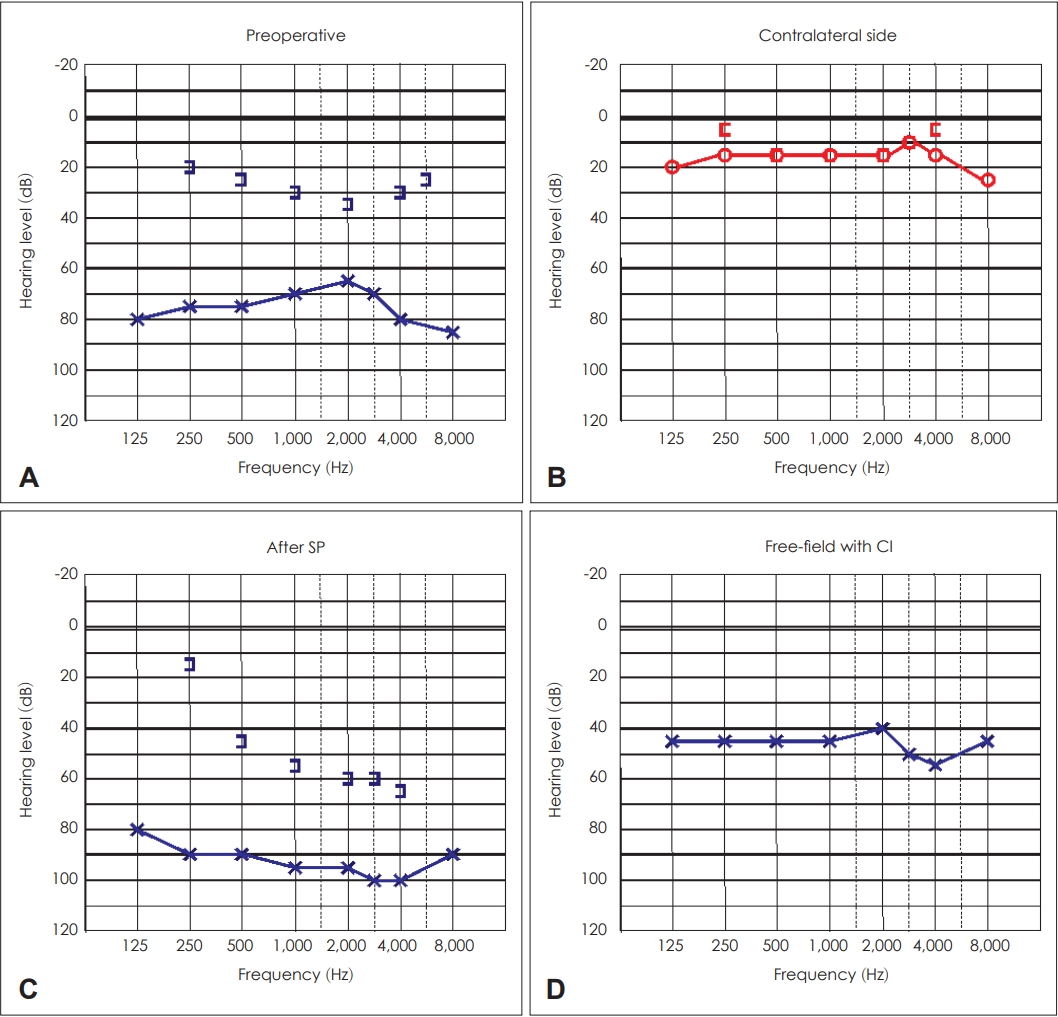
Fig.ô 4.
Pure tone audiograms of Case 2. A: Preoperative pure tone audiogram of the right ear depicting severe mixed hearing loss. B: The audiogram of the contralateral ear representing a mild mixed hearing loss except at the frequency of 125 Hz, where the threshold is 55 dB. C: The audiogram of the left ear after subtotal petrosectomy (SP) showing profound mixed hearing loss. D: Free-field pure tone audiogram illustrating mild to moderate hearing loss 30 months following implantation. CI, cochlear implantation.

Fig.ô 5.
Pure tone audiograms of the left ear of Case 3. A: Preoperative pure tone audiogram exhibiting almost complete deafness with a bone conduction worse than 65 dB above 500 Hz. B: Free-field pure tone audiogram showing a mild hearing loss 43 months following surgery. CI, cochlear implantation.
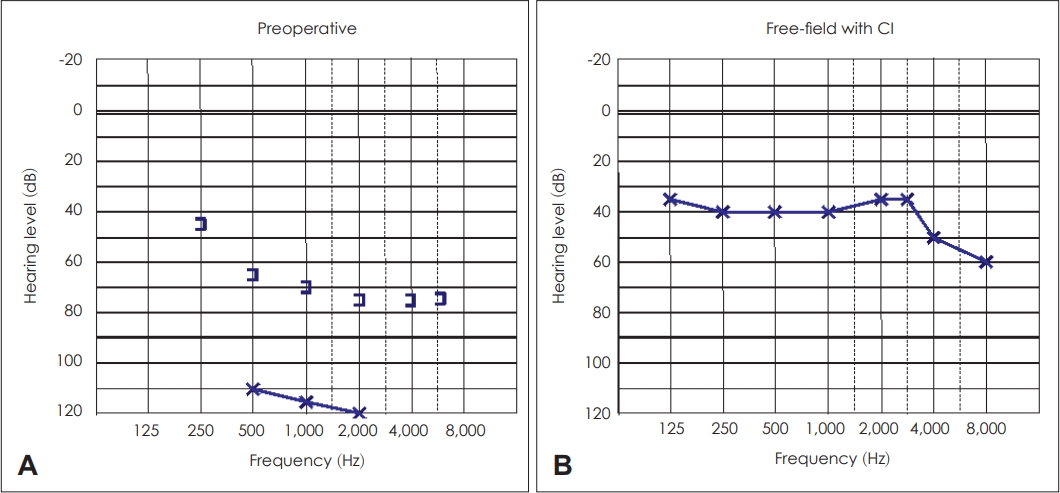
Fig.ô 6.
Pure tone audiograms of the right ear of Case 3. A: Preoperative pure tone audiogram demonstrating almost completely deafness with a bone conduction worse than 65 dB above 500 Hz. B: The audiogram following subtotal petrosectomy (SP) representing no deterioration in bone conduction. C: Free-field audiogram depicting a mild hearing loss 33 months following implantation. CI, cochlear implantation.
Tableô 1.
Demographic data of patients who underwent subtotal petrosectomy with simultaneous or sequential CI
REFERENCES
1. Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One 2012;7:e36226.



2. Verhoeff M, van der Veen EL, Rovers MM, Sanders EA, Schilder AG. Chronic suppurative otitis media: a review. Int J Pediatr Otorhinolaryngol 2006;70:1ã12.


3. Rosito LS, Netto LS, Teixeira AR, da Costa SS. Sensorineural hearing loss in cholesteatoma. Otol Neurotol 2016;37:214ã7.


4. Singh B, Maharaj TJ. Radical mastoidectomy: its place in otitic intracranial complications. J Laryngol Otol 1993;107:1113ã8.


5. Kerckhoffs KG, Kommer MB, van Strien TH, Visscher SJ, Bruijnzeel H, Smit AL, et al. The disease recurrence rate after the canal wall up or canal wall down technique in adults. Laryngoscope 2016;126:980ã7.


6. Vercruysse JP, De Foer B, Somers T, Casselman JW, Offeciers E. Mastoid and epitympanic bony obliteration in pediatric cholesteatoma. Otol Neurotol 2008;29:953ã60.


7. Maassen MM, LûÑwenheim H, Pfister M, Herberhold S, Jorge JR, Baumann I, et al. Surgical-handling properties of the titanium prosthesis in ossiculoplasty. Ear Nose Throat J 2005;84:142ã4. 147ã9.


8. Macnamara M, Phillips D, Proops DW. The bone anchored hearing aid (BAHA) in chronic suppurative otitis media (CSOM). J Laryngol Otol Suppl 1996;21:38ã40.


9. Arndt S, Aschendorff A, Laszig R, Beck R, Schild C, Kroeger S, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol 2011;32:39ã47.


10. Pawlowski KS, Wawro D, Roland PS. Bacterial biofilm formation on a human cochlear implant. Otol Neurotol 2005;26:972ã5.


12. Prasad SC, Roustan V, Piras G, Caruso A, Lauda L, Sanna M. Subtotal petrosectomy: surgical technique, indications, outcomes, and comprehensive review of literature. Laryngoscope 2017;127:2833ã42.


13. Coker NJ, Jenkins HA, Fisch U. Obliteration of the middle ear and mastoid cleft in subtotal petrosectomy: indications, technique, and results. Ann Otol Rhinol Laryngol 1986;95(1 Pt 1):5ã11.


14. Fayad JN, Tabaee A, Micheletto JN, Parisier SC. Cochlear implantation in children with otitis media. Laryngoscope 2003;113:1224ã7.

15. Axon PR, Mawman DJ, Upile T, Ramsden RT. Cochlear implantation in the presence of chronic suppurative otitis media. J Laryngol Otol 1997;111:228ã32.


16. Hellingman CA, Geerse S, de Wolf MJF, Ebbens FA, van Spronsen E. Canal wall up surgery with mastoid and epitympanic obliteration in acquired cholesteatoma. Laryngoscope 2019;129:981ã5.


17. Himi T, Harabuchi Y, Shintani T, Yamaguchi T, Yoshioka I, Kataura A. Surgical strategy of cochlear implantation in patients with chronic middle ear disease. Audiol Neurootol 1997;2:410ã7.


18. Vincenti V, Pasanisi E, Bacciu A, Bacciu S, Zini C. Cochlear implantation in chronic otitis media and previous middle ear surgery: 20 years of experience. Acta Otorhinolaryngol Ital 2014;34:272ã7.


19. Yoo MH, Park HJ, Yoon TH. Management options for cochlear implantation in patients with chronic otitis media. Am J Otolaryngol 2014;35:703ã7.


20. Olgun L, Batman C, Gultekin G, Kandogan T, Cerci U. Cochlear implantation in chronic otitis media. J Laryngol Otol 2005;119:946ã9.


21. Grinblat G, Vlad D, Caruso A, Sanna M. Evaluation of subtotal petrosectomy technique in difficult cases of cochlear implantation. Audiol Neurootol 2020;25:323ã35.


22. Szymaéski M, Ataide A, Linder T. The use of subtotal petrosectomy in cochlear implant candidates with chronic otitis media. Eur Arch Otorhinolaryngol 2016;273:363ã70.



23. Grinblat G, Prasad SC, Fulcheri A, Laus M, Russo A, Sanna M. Lateral skull base surgery in a pediatric population: a 25-year experience in a referral skull base center. Int J Pediatr Otorhinolaryngol 2017;94:70ã5.


24. Lee S, Lee JB, Chung JH, Park KW, Choi JW. Surgical outcomes of simultaneous cochlear implantation with subtotal petrosectomy. Auris Nasus Larynx 2020;47:943ã9.


25. Yan F, Reddy PD, Isaac MJ, Nguyen SA, McRackan TR, Meyer TA. Subtotal petrosectomy and cochlear implantation: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2020;147:1ã12.
26. Ketelslagers K, Somers T, De Foer B, Zarowski A, Offeciers E. Results, hearing rehabilitation, and follow-up with magnetic resonance imaging after tympanomastoid exenteration, obliteration, and external canal overclosure for severe chronic otitis media. Ann Otol Rhinol Laryngol 2007;116:705ã11.


27. Free RH, Falcioni M, Di Trapani G, Giannuzzi AL, Russo A, Sanna M. The role of subtotal petrosectomy in cochlear implant surgery--a report of 32 cases and review on indications. Otol Neurotol 2013;34:1033ã40.


28. Gerlinger I, MolnûÀr K, Nepp N, Tû°th I, Tû°th T, Szanyi I, et al. Subtotal petrosectomy-indications, surgical technique, experiences in Pûˋcs. Orv Hetil 2020;161:544ã53.

29. Vashishth A, Fulcheri A, Prasad SC, Dandinarasaiah M, Caruso A, Sanna M. Cochlear implantation in chronic otitis media with cholesteatoma and open cavities: long-term surgical outcomes. Otol Neurotol 2018;39:45ã53.









