Introduction
In patients with recurrent, intractable hearing disturbance or vertigo despite adequate medication, the possibility of tumors of cerebellopontine angle (CPA) or internal auditory canal (IAC) should be investigated. The most common tumors at these sites are vestibular schwannomas (VSs), followed by CPA meningioma [
1]. VSs are slow-growing, histologically benign tumors that are typically unilateral and sporadic; however, bilateral VSs have been reported in patients with neurofibromatosis type II (NF-II) [
2]. Despite being low risk for malignant transformation, VS can result in gradual, irreversible sensorineural hearing loss over time and persisting disequilibrium and vertigo [
3]. Therefore, early suspicion, detection, and management of VS could benefit patients by preventing further deterioration of their hearing and vestibular function.
Since the 1970s, the clinical efficacy of the auditory brainstem response (ABR) for the detection of VS has been compared with other diagnostic modalities [
4-
8]. Currently, gadolinium-enhanced temporal bone magnetic resonance imaging (Gd-TBMRI) is accepted as the ŌĆ£gold standardŌĆØ method for the detection of VS [
3,
9]. However, its low cost efficiency causes many physicians to hesitate to recommend MR upon diagnosing idiopathic unilateral sensorineural hearing loss or persisting/recurrent vertigo despite adequate medication [
4,
10,
11]. We, therefore aimed to investigate the diagnostic yield of abnormal ABR in patients with radiologically proven VS in a tertiary medical center setting, and thereby define the role of ABR in the detection of VS.
Results
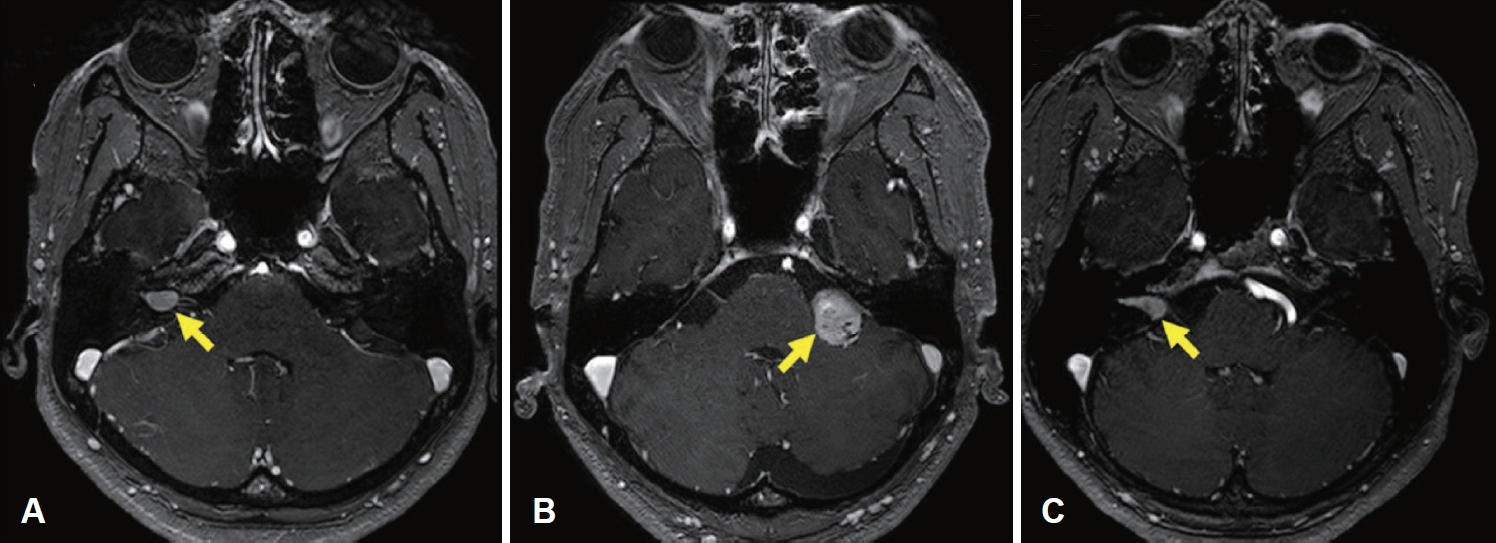
Patient clinical and demographic data are presented in
Table 2. The mean age was 50.4 years (range 12-78), and the male-to-female ratio was 2:1. The mean long-axis tumor diameter measured on Gd-TBMRI was 16.4 mm (range 4-46), with 15 cases (37.5%) confined to the IAC. The average PTA
4 was a mean value and a standard deviation (SD) of 45.7┬▒32.7 dB HL, with a mean WRS and SD of 69.3┬▒36.9%. Patients with serviceable hearing were observed in 24 (60.0%) cases.
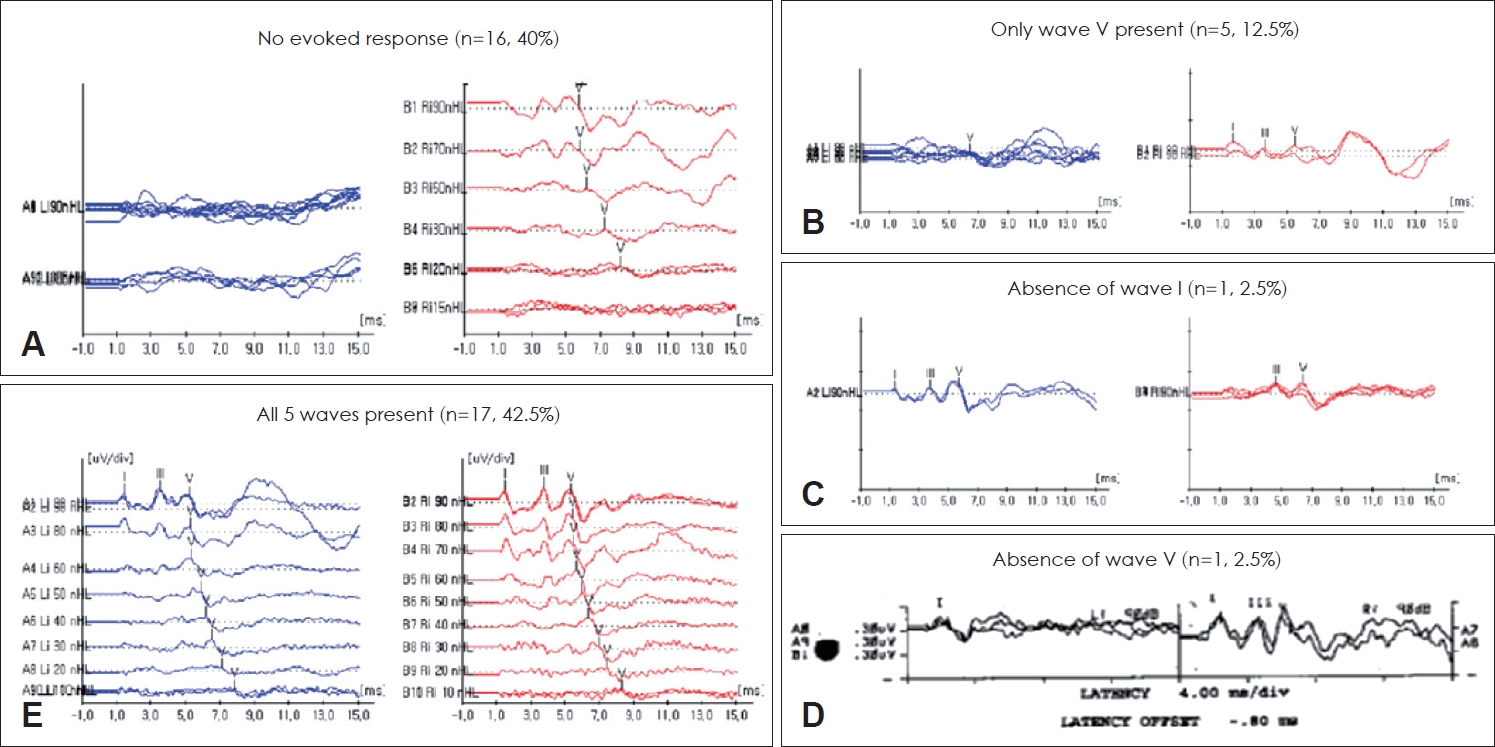
Overall sensitivity of ABR for the detection of VS was 85.0%. Six patients (15.0%) were classified as normal according to the ABR criteria shown in
Table 1. In patients who showed an abnormal ABR waveform pattern, no response was noted in 16 patients (40.0%) at 90 dB SPL (
Fig. 2A), five patients (12.5%) had only wave V present (
Fig. 2B), one patient (2.5%) showed the absence of wave I (
Fig. 2C), and one patient (2.5%) with the absence of wave V (
Fig. 2D).
Preservation of all five waves was observed in 17 (42.5%) of 40 patients (
Fig. 2E), and 11 patients (32.4%) were assessed as having an abnormal ABR according to the diagnostic criteria using the interpeak latency and the intraaural differences. The highest VS sensitivity occurred when intraaural latency differences between wave I and V were more than 0.2 ms (47.1%) (
Table 3). Tumor size, ratio of micro to macro-tumors, and patientsŌĆÖ hearing ability in each six cut-off diagnostic criterion for the detection of VS were described in
Table 3.
Tumor size was significantly smaller (8.7┬▒3.3 mm) in patients with normal ABR than in patients with abnormal ABR (
Table 4). Although not statistically significant, the lowest sensitivity was detected in intracanalicular VS and tumors smaller than 10 mm in long-axis diameter (73.3% and 66.7%, respectively) compared with VS confined to CPA and tumors greater than 30 mm in long-axis diameter, both revealing sensitivity of 100.0%. The average of pure tone thresholds was higher in patients with abnormal ABR (48.1┬▒32.9 dB HL) than patients with normal ABR (27.9┬▒20.5 dB HL), although the difference was not statistically significant between the two groups. There was no significant difference in sensitivity between AAO-HNS Class D and Class A (100% and 76.9%, respectively). However, the mean WRS of patients with normal ABR (98.0%) was statistically different from patients with abnormal ABR (66.1%) (
p=0.027).
In 24 VS patients who had serviceable hearing (AAO-HNS class A or B), 19 patients showed abnormal ABR, showing sensitivity of 79.2 % (
Table 5). The mean tumor size of abnormal ABR patient group showed 15.1┬▒9.4 mm, showing a significant difference with the normal ABR VS patients (
p=0.040). The portion of macrotumor showed 20% in the normal ABR group, whereas 73.7% patients had macrotumor in the abnormal ABR group, showing a significant difference (
p=0.047).
Discussion
With the emergence of Gd-TBMRI in the 1980s, early detection of small VS larger than 3 mm had become possible, thereby making Gd-TBMRI the gold standard modality for the diagnosis of VS [
13]. In addition, the importance of early detection of VS before the onset of severe, irreversible symptoms such as profound hearing loss, vertigo, and facial palsy has been discussed by many researchers over recent decades [
2,
3,
14].
The management of VSs has become more conservative over time, with strategies including wait-and-scan becoming widely practiced. However, surgeons should bear in mind that certain patients could be salvaged with early VS treatments such as microsurgical resection and radiosurgery [
3,
15]. Early detection of VSs allows for more comprehensive therapeutic options to be available for patients, which would not be possible in cases of missed or delayed diagnosis. Therefore, a need for precise diagnosis of symptomatic patients with VS is essential.
Historically, several diagnostic modalities outside of Gd-TBMRI have been suggested to detect retrocochlear pathology. Kanzaki, et al. [
7] observed a poor WRS of 36.7%, and a low WRS, despite preserved PTA threshold, in patients with VS. Jerger, et al. [
6] additionally reported a 13% sensitivity of speech reception threshold (SRT) in patients with VS, whereas other researchers reported a sensitivity of 77% [
7]. Godey, et al. [
16] noted 84% sensitivity of SRT and 86% sensitivity of caloric vestibular response (CVR), totaling up to 98% sensitivity when all three SRT, CVR, ABR are used in combination for the detection of VS.
A recent meta-analysis reported that ABR shows an overall sensitivity of over 93% and specificity of over 82% [
17]. The abnormality of ABR for the detection of VS was assessed with the presence of disrupted waveform and the interpeak and intraaural latency differences in all of the previous studies, with a minor variation in each study [
9,
18]. Chandrasekhar, et al. [
5] highlighted the correlation between the size of VS with a diagnostic yield of ABR, showing 100% sensitivity in VS larger than 30 mm, whereas 83.1% sensitivity was obtained in VS smaller than 10 mm. However, Wilson, et al. [
8] have shown 4% and 33% false-negative predictability of ABR in extracanalicular and intracanalicular VS, respectively. These data align with our findings, which show an overall sensitivity of 85.0%; the lowest sensitivity was found in small, intracanalicular VS (66.7%-73.3%) and the highest sensitivity in large VS confined to the CPA (100.0%).
Our results showed the highest sensitivity when the interaural latency difference of wave V >0.2 ms and the interaural difference of interpeak latency between wave I and V >0.2 ms was used (43.8% and 47.1%, respectively). In previous articles, the sensitivity of these two criteria showed a range of 66%-97% [
15]; however, imaging techniques were not universalized at their time of publication (1990s). Therefore, an early detection rate could be much lower than in our study, determined as 90% of extracanalicular VS, with a mean size of 26 mm according to Godey, et al. [
16]. In our data, 37.5% of patients had an intracanalicular VS, with an average tumor size of 16.4 mm, which potentially explains the difference in the sensitivity of ABR between the previous studies and our own.
Grimes and Schulz [
19] and Shickle and Chadwick [
20] proposed a set of criteria that would qualify a diagnostic modality with good validity in screening for a specific disease: 1) the test should be performed with ease; 2) the test should have a high costŌĆōbenefit ratio; 3) the disease should be detected easily in the early stage; 4) full recovery of the disease should be expected with early detection, and 5) the sensitivity of the test should have a high yield in the majority of the patients. The ABR is an easy, non-invasive, relatively inexpensive diagnostic tool for the detection of VS, particular for those that are large and extracanalicular. Denmark and India had previously omitted ABR at VS screening to reduce expenditure (40,000 EURO/year; 1,200 USD/patient, respectively) [
11,
21]. In South Korea, the cost for the Gd-TBMRI in 2017 was 830,000 Korean Won (approx. 740.41 USD), compared with 128,076 Korean Won (approx. 114.25 USD) for ABR threshold test, and less than 50,000 Korean Won (approx. 44.60 USD) for the ABR with bilateral 90 dBnHL click stimuli for the screening of VS. Approximately, more than 700 USD per every patient when ABR with 90 dBnHL click stimuli replaced Gd-TBMRI at VS screening.
As mentioned in many previous studies [
22-
24], patients with confirmed VS are often carefully observed over time, due to low rate of malignant transformation, slow growth, and life-threatening complication. Especially in micro-VS patients with serviceable hearing, treatment of VS with surgery of gammaknife radiosurgery bears the risk of hearing deterioration. Moreover, these treatment modality possesses the risk for facial palsy and vestibular dysfunction, which are two very rare clinical findings in the natural course of VS. Therefore, it would be important to detect the micro to macro-transformation of VS in the clinical practice. Our data show low sensitivity of ABR in the serviceable hearing group (AAO-HNS class A or B) (76.9% and 81.8%, respectively), compared with nonserviceable hearing group, showing 100.0% sensitivity. However, it should be noticed that in serviceable hearing group, the portion of macrotumor was significantly higher in patients with abnormal ABR findings then patients with normal ABR findings. These results support ABR may aid in screening macrotumors in patients with serviceable hearing, thereby providing an adequate timing for surgical intervention.
To our knowledge, no published studies have previously reported on the diagnostic validity of ABR for the screening of VS considering the patientŌĆÖs initial hearing status with tumor size. Although our data propose an incongruity of ABR in VS screening in a linear fashion with previous studies, our study bears some limitations: 1) ABR was conducted in a limited number of patients due to the retrospective nature of the study, and 2) the information on patients who had taken ABR without radiological evidence of VS is lacking. Therefore, the specificity and costŌĆōbenefit analysis could not be performed.
Our findings support ABR as a valuable tool for the detection of large, extracanalicular tumors; however, is runs a risk for false-negative results in small, intracanalicular tumors. Therefore, Gd-TBMRI should remain as the gold standard diagnostic modality in VS screenings. However, in patients with preserved hearing, ABR may act as a cost-effective screening modality to detect large VS, enabling the physician to recommend a Gd-TBMRI along with more active treatment plan.