|
 |
- Search
| J Audiol Otol > Volume 28(2); 2024 > Article |
|
Abstract
Background and Objectives
Materials and Methods
Results
Supplementary Materials
Supplementary Material 2.
Supplementary Material 3.
Supplementary Material 4.
Notes
Author Contributions
Conceptualization: Maria Renata José. Data curation: Maria Renata José, Jéssica da Silva Ortega, Camila de Castro Corrêa, Karinna Veríssimo Meira Taveira, Cristiano Miranda de Araujo. Formal analysis: Maria Renata José, Camila de Castro Corrêa, Karinna Veríssimo Meira Taveira, Cristiano Miranda de Araujo. Investigation: Maria Renata José, Jéssica da Silva Ortega, Jordana Batista Correia Baran. Methodology: Maria Renata José, Jéssica da Silva Ortega, Camila de Castro Corrêa, Karinna Veríssimo Meira Taveira, Cristiano Miranda de Araujo. Project administration: Maria Renata José, Cristiano Miranda de Araujo. Supervision: Maria Renata José, Cristiano Miranda de Araujo. Validation: Maria Renata José, Débora Lüders, Claudia Giglio de Oliveira Gonçalves, Cristiano Miranda de Araujo. Visualization: Maria Renata José, Jéssica da Silva Ortega, Jordana Batista Correia Baran, Débora Lüders, Claudia Giglio de Oliveira Gonçalves, Bianca Simone Zeigelboim, Camila de Castro Corrêa, Karinna Veríssimo Meira Taveira, José Fernando Polanski, Rosane Sampaio Santos, Cristiano Miranda de Araujo. Writing—original draft: Maria Renata José, Jéssica da Silva Ortega, Débora Lüders, Camila de Castro Corrêa, Karinna Veríssimo Meira Taveira, Cristiano Miranda de Araujo. Writing—review & editing: Maria Renata José, Jéssica da Silva Ortega, Jordana Batista Correia-Baran, Débora Lüders, Claudia Giglio de Oliveira Gonçalves, Bianca Simone Zeigelboim, Karinna Veríssimo Meira Taveira, José Fernando Polanski, Rosane Sampaio Santos, Cristiano Miranda de Araujo. Approval of final manuscript: all authors.
Table 1.
| Study | Country | Condition or disease | Medication | Sample, n | Group with the disease (exposed with CQ or HCQ / unexposed), n | Group without the disease (unexposed), n | Average age (yr) | Tests used for hearing assessment |
Results of audiological assessments |
Main conclusions | Study design | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group with the disease | Group without the disease | |||||||||||
| Aurélio, et al. [27] | Brazil | Malaria (during pregnancy) | CQ (VO) | 35 | 34/1 | None | Mean gestational age 38-6/7 weeks (range: 30 to 41 weeks) | OAE | Group of mothers with gestational malaria (n=35) | None | The prevalence of hearing loss in newborns with mothers who contracted malaria and underwent drug treatment during pregnancy was 3% (n=1). | Cross Sectional |
| 25 mg/total dose | ABR | |||||||||||
| D0: 10 mg/kg | ||||||||||||
| D1: 7.5 mg/kg | - Exposed to CQ (n=34) | |||||||||||
| D2: 7.5 mg/kg | One newborn was diagnosed with retrocochlear hearing loss. | |||||||||||
| 5 mg/kg/wk (300 mg maximum dose) for 3 months (continuation of chemoprophylaxis after this period may be necessary in some cases) | ||||||||||||
| Barrenäs [23] | Sweden | RA | CQ (there is no dose) | 12 | 12/0 | None | G1: 41.8±17.7 | Békesy audiometry | Average pre-exposure to noise and using CQ | None | CQ increased the susceptibility to temporary change in the post-exposure noise threshold, which was more pronounced in young subjects with large amounts of dermal melanin. | Cohort |
| G1: subjects with less melanin | G2: 47.7±13.8 | G1: 18.2±15.1 | ||||||||||
| G2: 13.7±6.7 | ||||||||||||
| G2: subjects with greater amount of melanin | Average post exposure to noise (105 dB SPL for 10 minutes) and using CQ: | |||||||||||
| G1: 24.5±14.8 | ||||||||||||
| G2: 19.7±7.5 | ||||||||||||
| Average pre-exposure to noise after cessation of CQ use (4 weeks): | ||||||||||||
| G1: 19.2±18.1 | ||||||||||||
| G2: 17.8±8.3 | ||||||||||||
| Average post exposure to noise (105 dB SPL for 10 minutes) after cessation of CQ use (4 weeks): | ||||||||||||
| G1: 24.7±17.7 | ||||||||||||
| G2: 21.7±8.2 | ||||||||||||
| Bernard [20] | Canada | RA and SLE | CQ - 205 mg daily dose of CQ (in combination with non-salicylate anti-inflammatory drugs) | 168 | 74/0 | 94 | AR and SLE Group: median of 39 years (range 18 to 50 years) | PTA | AR and SLE group (n=74) | Group without the disease (n=94) | Chloroquine phosphate leads to ototoxic side effects that are reversible if the medication is stopped. Occurrence of a case of irreversible hearing loss due to continuous treatment. | Cohort |
| RA: 70 participants | VA | - Exposed to combined CQ (n=74) | ||||||||||
| SLE: 4 participants | ABR | ABR wave I (in ms) in the 1st, 5th, and 12th month of treatment: 1.34; 1.34; and 1.36 | Mean and SD wave I of the ABR (in ms): 1.37±0.04 | |||||||||
| Group without the disease: range from 19 to 31 years old | ABR wave III (in ms) in the 1st, 5th, and 12th month of treatment: 3.49; 3.75; and 3.48 | Mean and SD wave III of the ABR (in ms): 3.51±0.06 | ||||||||||
| ABR wave V (in ms) in the 1st, 5th, and 12th month of treatment: 5.50; 5.83; and 5.54 | Mean and SD wave V of the ABR (in ms): 5.51±0.04 | |||||||||||
| ABR interpeak interval I–III (in ms) in the 1st, 5th, and 12th month of treatment: 2.15; 2.41; and 2.16 | Mean and SD interpeak interval I-III of the ABR (in ms): 2.14±0.10 | |||||||||||
| ABR III-V interpeak interval (in ms) in the 1st, 5th, and 12th month of treatment: 2.01; 2.07; and 2.06 | Mean and SD ABR III-V interpeak interval (in ms): 2.00±0.10 | |||||||||||
| ABR I-V interpeak interval (in ms) in the 1st, 5th, and 12th month of treatment: 4.16; 4.48; and 4.16 | Mean and SD interpeak I-V interval of ABR: 4.14±0.21 | |||||||||||
| 13 participants (all with RA and with PTA results within normal limits) using CQ had altered results in the ABR; 12 of these after treatment suspension, in a period of 12 to 16 months, presented results within the normality standard in the ABR, and one participant who needed to continue the treatment with the CQ presented bilateral hearing loss, of the sensorineural type, of moderate degree. | PTA, VA, and tympanometry within normal standards | |||||||||||
| Borba, et al. [18] | Brazil | SLE (during pregnancy) | CQ | 19 | 9/10 | None | Group of children exposed to CQ during pregnancy: 7.5±4.3 | PTA | Group of children exposed to CQ during pregnancy (n=9) | None | Intrauterine exposure of children to chloroquine diphosphate in this study was not associated with hearing loss. | Cross Sectional |
| 250 mg/day - four pregnant women in the first trimester and five throughout pregnancy | Group of children not exposed to CQ during pregnancy: 12.3±7.2 | Average PTA (Frequencies of 250, 500, and 1,000 Hz): 11.4±4.5 | ||||||||||
| Average PTA (Frequencies of 2,000, 4,000, and 8,000 Hz): 8.5±5.0 | ||||||||||||
| Group of children not exposed to chloroquine during pregnancy (n=10) | ||||||||||||
| Average PTA (Frequencies of 250, 500, and 1,000 Hz): 11.9±3.0 | ||||||||||||
| Average PTA (Frequencies of 2,000, 4,000, and 8,000 Hz): 7.4±3.6 | ||||||||||||
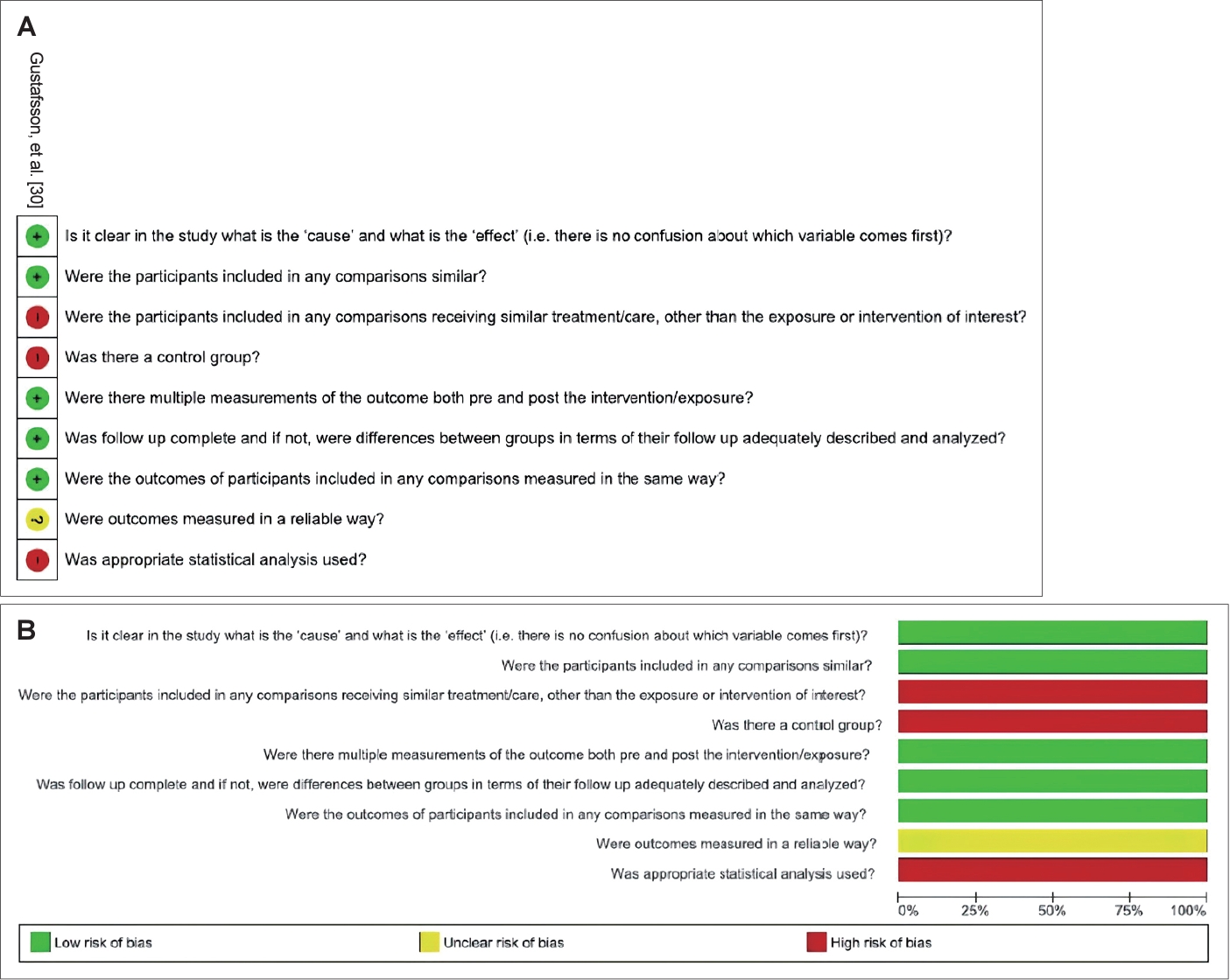
| Gustafsson, et al. [30] | Uruguay | Healthy volunteers | CQ | 11 | 0 | 11 | Between 20 to 36 | PTA | None | Group without disease (n=11) | Conventional PTA and HFA did not reveal conclusive signs of ototoxicity. In two cases, there were significant differences in HFA, but not in conventional PTA. | Non-randomized clinical trial |
| 3 doses (intravenous infusion, oral solution and tablet) of 300mg at intervals of at least 56 days | HFA | Exposed of CQ (n=11) | ||||||||||
| None participant (n=11) showed changes in conventional PTA. | ||||||||||||
| In HFA, the mean threshold values for 10, 11, 12 and 13 kHz did not show a significant increase after the administration of CQ. | ||||||||||||
| Halligan, et al. [26] | USA | RA | HCQ (there is no dose) | 59 | 6/23 | 30 | RA group: range 40 to 69 | PTA | RA group (n=29) | Group without disease (n=30): 14 participants had hearing loss. | The use of medication was not the focus of the study, but there was an increase in the number of HCQ users with hearing impairments compared to those who had hearing impairments and did not use the medication. | Cross Sectional |
| Group without disease: range 40 to 69 | VA | - Exposed to HCQ (n=6): 6 participants had hearing loss. | ||||||||||
| Immittance measurements | - Not exposed to HCQ (n=23): 11 had hearing loss. | |||||||||||
| OAE transient | ||||||||||||
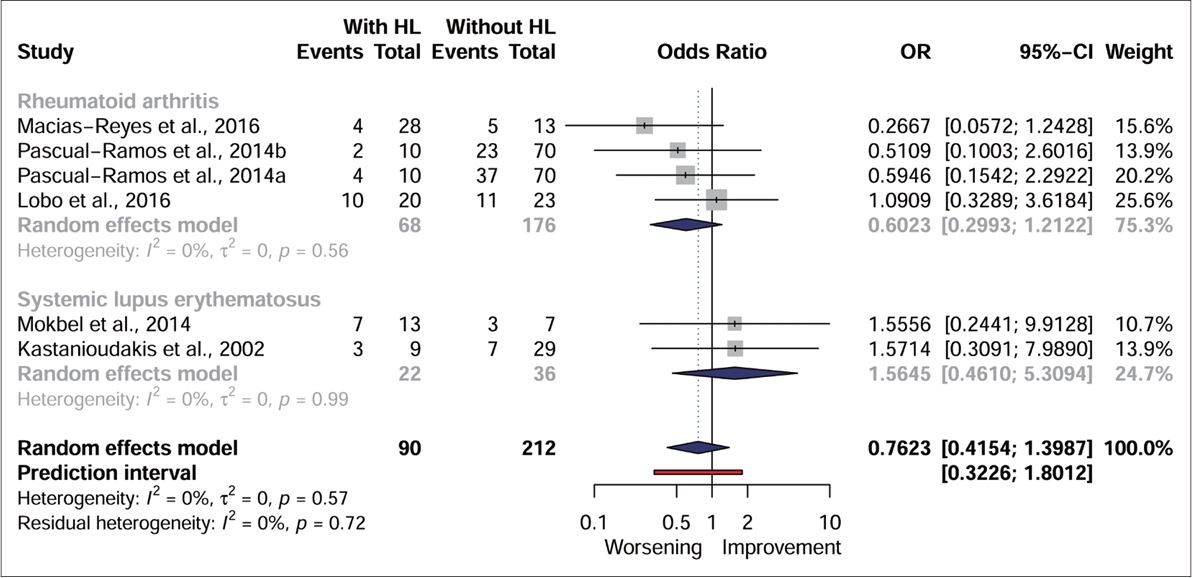
| Kastanioudakis, et al. [19] | Greece | SLE | HCQ (in combination with steroids, Nifedipine, Azathioprine, Cyclosporine-A, Methotrexate and Non-steroidal anti-inflammatory drugs; there is no dose) | 88 | 10/28 | 50 | SLE group: 47.86±12.69 | PTA | SLE group (n=38) | Group without disease (n=50) | No significant differences were found between the two groups, with and without hearing loss, in relation to drug therapy. | Cross sectional |
| VA | - Exposed to combined HCQ (n=10) | Number of participants with hearing impairment is not mentioned. | ||||||||||
| Group without disease: paired with SLE group | Immittance measurements | |||||||||||
| ABR (if there was suspicion of retrocochlear alteration) | 3 participants had hearing loss. | |||||||||||
| Kokong, et al. [28] | Nigeria | Drug-induced ototoxicity | CQ (there is no dose) | 156 | 22/134 (exposed to other medications) | None | 32.1±30.7 (range: 5-85) | PTA | Group with varied diseases (n=156) | None | CQ was the second main drug involved in the study and its ototoxicity had a prevalence of 14.1%. | Cross sectional |
| Tympanometry | - Exposed to CQ (n=22; 44 ears) | |||||||||||
| 35 ears had hearing alterations (different degrees) associated with CQ ototoxicity. | ||||||||||||
| Lobo, et al. [22] | Brazil | RA | CQ (in combination with Methotrexate and/or leflunomide, corticosteroid and non-steroidal anti-inflammatory; there is no dose) | 66 | 21/22 | 23 | RA group: 48.86±7.35 | PTA | RA group (n=43) | Group without disease (n=23) | No significant differences were found between subjects with or without hearing impairment in relation to the use of CQ. | Cross sectional |
| Group without disease: 47.04±5.69 | VA | - Exposed to CQ (n=21) | Normal hearing: 16 participants | |||||||||
| Immittance measurements | 10 participants had hearing loss. | Conductive hearing loss: 1 participant | ||||||||||
| OAE | Sensorineural hearing loss: 6 participants | |||||||||||
| Macias-Reyes, et al. [24] | Mexico | RA | CQ (in possible combination with methotrexate, sulfasalazine, azathioprine and prednisone; there is no dose) | 41 | 9/32 | None | RA group: 46.5±10.2 | PTA | RA group (n=41) | None | There was no significant difference between participants who had or not hearing loss and who were using the CQ. | Cross sectional |
| Tympanometry | - Exposed to CQ (n=9) | |||||||||||
| 4 participants had sensorineural hearing loss. | ||||||||||||
| Maciaszczyk, et al. [17] | Poland | SLE | CQ (in combination with corticosteroids; there is no dose) | 65 | 23/12 | 30 | SLE group: 47.8±11,3 | PTA | SLE group (n=35) | Group without the disease (n=30) | Logistic regression analysis did not indicate the administration of CQ as a probable cause of hearing alterations. | Cross sectional |
| Group without disease: 47.8±9.8 | VA | -Exposed to CQ (n=23) | Number of participants with hearing impairment is not mentioned. | |||||||||
| Immittance measurements | 8 participants had sensorineural hearing loss. | |||||||||||
| ABR | ||||||||||||
| Mokbel, et al. [16] | Egypt | SLE | HCQ (in combination with steroids and azathioprine) | 40 | 10/10 | 20 | SLE group: 28.8±9.36 (range: 20 to 48) | PTA | SLE group (n=20) | Group without the disease (n=20) | There was no significant difference in the medications received between those with and without hearing loss. However, it was observed that hearing level was significantly lower in those who received Azathioprine and HCQ compared to those who did not. | Cross sectional |
| Dose between 200 at 400 mg/day (mean 340±96.6) | Group without disease: 33±8.03 (range: 22 to 49) | VA | -Exposed to combined HCQ (n=10) | All subjects in the control group (100%) had normal hearing. | ||||||||
| Immittance measurements | 7 women using HCQ combined with other drugs had hearing disorders. | |||||||||||
| Obasikene, et al. [29] | Nigeria | Drug-Induced Ototoxicity | CQ (there is no dose) | 79 | 5/74 (exposed to other medications) | None | 31-40 years and±1.89 (range: 6 months to 70 years) | PTA | Group with varied diseases (n=79) | None | Of the drugs identified in the study, CQ was listed as the third main drug causing hearing loss, with a prevalence of 6.3% in the sample. | Cross sectional |
| Verbal speech and distraction method (children of less than 4 years) | -Exposed to CQ (n=5) | |||||||||||
| 5 ears had hearing loss. | ||||||||||||
| Pascual-Ramos, et al. [25] | Mexico | RA | CQ – Daily dose of 150 mg/day* | 104 | 79/25 | None | RA group: 43.4±13.3 | Conventional PTA (125 Hz to 8 kHz) | RA group (n=104) | None | There was no relationship between incidental hearing loss and drug treatment. | Cohort |
| HFA (9 kHz to 18 kHz) | -Exposed to combined CQ (n=79) | |||||||||||
| At the start of the study: Combination of Methotrexate and CQ (n=49): 4 participants had hearing loss (one participant taking a combination of corticosteroids, and another taking a combination of | ||||||||||||
| non-steroidal anti-inflammatory drugs**). | ||||||||||||
| Combination of methotrexate, CQ and sulfasalazine (n=30): 2 participants had hearing loss. | ||||||||||||
| After one year follow-up: combination of methotrexate and CQ (n=49): 4 participants had hearing loss. | ||||||||||||
| Combination of methotrexate, CQ and sulfasalazine (n=30): one participant had hearing loss (under combined use of non-steroidal anti-inflammatory drugs**). | ||||||||||||
| Polanski, et al. [21] | Brazil | SLE | CQ: 16 participants | 84 | 37/6 | 41 | SLE group: 40.8±13.0; Exposed to medication: 46.4±12.7; | PTA | SLE group (n=43) | Group without the disease (n=41) | CQ and HCQ were considered safe drugs for the auditory system. | Cross sectional |
| HCQ: 21 participants | VA | - Exposed to CQ and HCQ (n=37) | ||||||||||
| Dose of 5.0 mg/kg/day of HCQ and 3.0 mg/kg/day of CQ | Unexposed: 40.9±13.9 | Tympanometry | PTA average: 8.75 (5.00-13.75) | PTA average: 10.00 (5.93-12.50) | ||||||||
| Group without disease: paired with SLE group | Tympanometry: type A curve (100%) | Tympanometry: type A curve (100%) | ||||||||||
| Usage time (yr): OH CQ - median of 5 (IQR= 2-9.5) and CQ - median of 10 (IQR=5.5-12.0) | 7 participants had sensorineural hearing loss. | Control group participants did not have hearing loss. | ||||||||||
| - Not exposed to CQ and HCQ (n=6) | ||||||||||||
| PTA average: 8.75 (5.31-13.44) | ||||||||||||
| Tympanometry: type A curve (100%) | ||||||||||||
| 3 participants had sensorineural hearing loss. | ||||||||||||
| Roverano, et al. [15] | Argentina | SLE | HCQ (in combination with azathioprine, corticosteroids, methotrexate, and cyclophosphamide) | 55 | 25/5 | 25 | SLE group: 35 (range 19-64) | PTA | SLE group (n=30) | Group without the disease (n=25) | When comparing the exposed group and the group not exposed to HCQ, no correlation was found between the presence of hearing impairment and treatment with HCQ. | Cross sectional |
| Group without disease: paired with SLE group | VA | - Exposed to combined HCQ (n=25) | 3 had hearing impairment (conductive type); and 1 had bilateral sensorineural hearing loss. | |||||||||
| Dose of 200 mg/day | 16 had sensorineural hearing loss. | |||||||||||
| - Not exposed to combined HCQ (n=5) | ||||||||||||
| 5 had sensorineural hearing loss. | ||||||||||||
| Subramaniam and Vaswani [3] | India | Malaria | CQ (paracetamol) | 30 | 30/0 | None | Range: 14-58 | PTA | Conventional PTA did not reveal any hearing loss after treatment with CQ. HFA identified mild bilateral hearing loss at 12 kHz in one subject and mild to moderate bilateral hearing loss at 8 kHz in another subject after treatment. In these two individuals, abnormal latencies of wave V in the ABR and abnormal responses of OAE after treatment were found. After a month of drug therapy, the results returned to normal. | None | CQ can be safely used in regular doses in the treatment of malaria as ototoxic side effects are very rare and reversible. | Cohort |
| Initial dose of 1,200 mg, followed by 4 doses of 600 mg every 12 hours | HFA | |||||||||||
| ABR | ||||||||||||
| OAE | ||||||||||||
ABR, auditory brainstem response; CQ, chloroquine; dB SPL, decibel sound pressure level; HCQ, hydroxychloroquine; HFA, high-frequency audiometry; IQR, interquartile range; mg, milligrams; ms, milliseconds; n, number; OAE, otoacoustic emissions; PTA, pure-tone audiometry; RA, rheumatoid arthritis; SD, standard deviation; SLE, systemic lupus erythematosus; VA, vocal audiometry; VO, via oral
REFERENCES
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 963 View
- 108 Download
-
Efficacy of the Digit-in-Noise Test: A Systematic Review and Meta-Analysis2022 January;26(1)